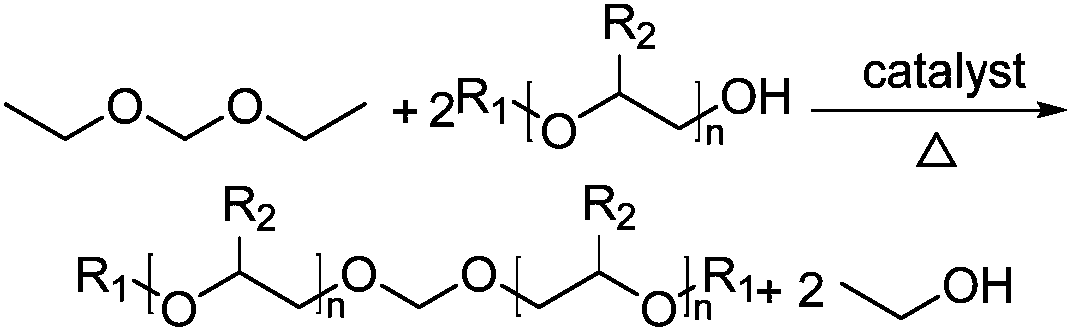
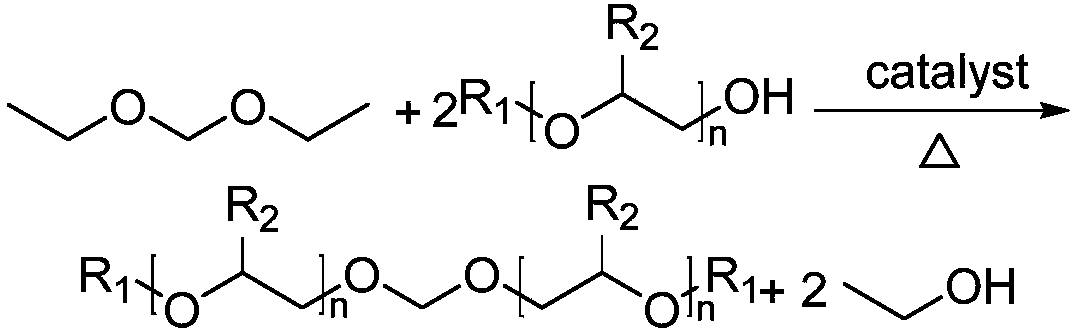
Bis-diol monoalkyl ether formaldehyde preparation method
A technology for diol monoalkyl ether and monoalkyl ether, which is applied in the field of preparing diglycol monoalkyl ether formal, can solve the problems such as difficulty in filtration and recovery of catalysts, reduce harm to human body, and improve product quality. Quality and effect of reducing waste water discharge
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Add 156.4g of diethoxymethane, 81.2g of diethylene glycol butyl ether, and 0.8g of phosphotungstic acid into a 500mL four-neck flask equipped with a thermometer, a magnetic rotor, and a condenser tube, stir and heat up to 80°C, and the reaction time is 90min Reaction finishes, and reactant is layered, reclaims lower layer catalyst and recycles; Upper layer liquid removes excess diethoxymethane and ethanol through decompression distillation, obtains light yellow two diethylene glycol butyl ether formal crude product 103.5g, gas chromatography According to analysis, the content of diethylene glycol butyl ether formal is 68.9%, and the content of diethylene glycol butyl ether is 11.8%. The crude product can be refined to obtain bisdiethylene glycol butyl ether formal with a purity greater than 98%.
Embodiment 2
[0027] Add 156.1g of diethoxymethane, 81.4g of diethylene glycol butyl ether, and 0.8g of phosphotungstic acid into a 500mL four-neck flask equipped with a thermometer, a magnetic rotor, and a condenser tube, stir and heat up to 75°C, and the reaction time is 90min Reaction finishes, and reactant is layered, reclaims lower layer catalyst and recycles; Upper layer liquid removes excess diethoxymethane and ethanol through decompression distillation, obtains light yellow two diethylene glycol butyl ether formal crude product 102.4g, gas chromatography According to analysis, the content of diethylene glycol butyl ether formal is 66.4%, and the content of diethylene glycol butyl ether is 13.5%. The crude product can be refined to obtain bisdiethylene glycol butyl ether formal with a purity greater than 98%.
Embodiment 3
[0029] Add 156.5g of diethoxymethane, 81.3g of diethylene glycol butyl ether, and 0.8g of phosphotungstic acid into a 500mL four-neck flask equipped with a thermometer, a magnetic rotor, and a condenser tube, stir and heat up to 70°C, and the reaction time is 90min Reaction finishes, and reactant is layered, reclaims lower layer catalyst and recycles; Upper layer liquid removes excess diethoxymethane and ethanol through decompression distillation, obtains light yellow two diethylene glycol butyl ether formal crude product 101.8g, gas chromatography According to analysis, the content of diethylene glycol butyl ether formal is 65.9%, and the content of diethylene glycol butyl ether is 14.1%. The crude product can be refined to obtain bisdiethylene glycol butyl ether formal with a purity greater than 98%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| density | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com