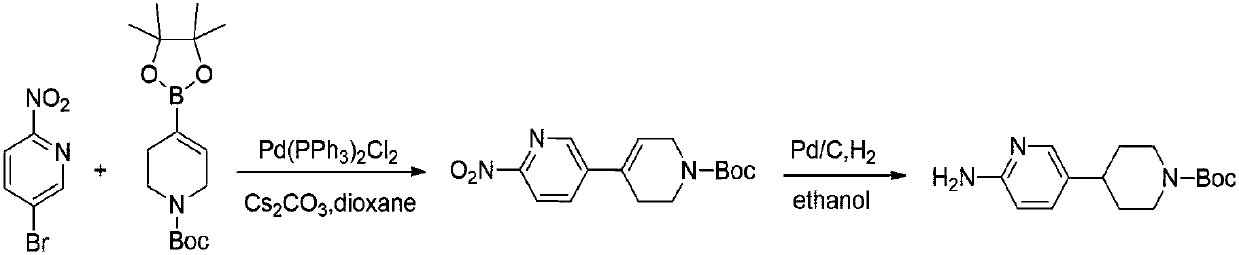
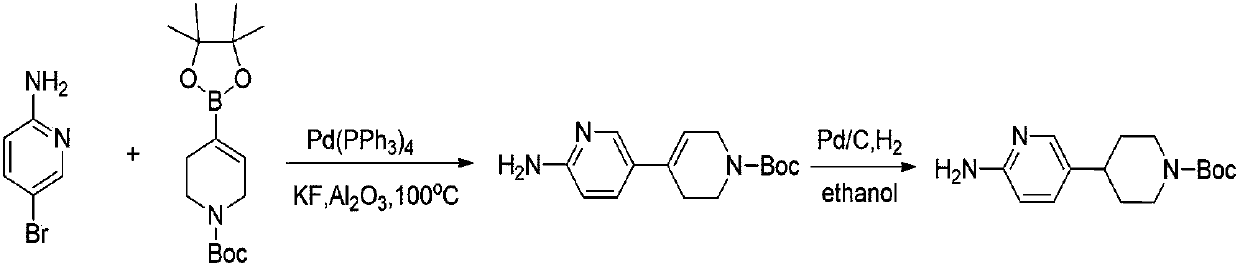
Preparation method of 4-(6-aminopyridine-3-radical) piperidine-1-tert-butyl formate
A technology of tert-butyl formate and aminopyridine, which is applied in the field of pharmaceutical chemical synthesis, can solve the problems of many reaction impurities, high price, low synthesis process yield, etc., and achieves the effects of reducing environmental pollution, reducing production cost and optimizing preparation process.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
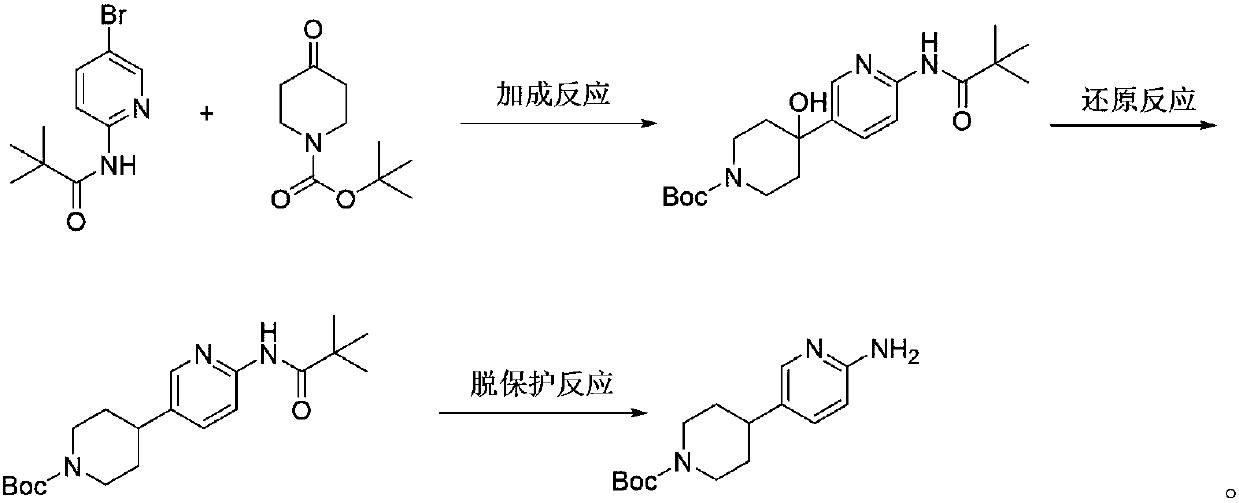
[0031] The first step: the synthesis of 4-(6-trimethylacetamido-3-pyridyl)-4-hydroxy-N-tert-butoxycarbonylpiperidine
[0032] At -70°C, n-butyl lithium (37.2 mL, 93 mmol) in n-hexane (2.5 N) was slowly added to N-(5-bromo-pyridine-2-)-2,2-dimethylpropanamide ( 7.94 g, 31 mmol) in diethyl ether (80 mL), and the reaction mixture was stirred at this temperature for 1 hour. Diethyl ether (60 mL) of N-tert-butoxycarbonyl-4-piperidone (6.15 g, 31 mmol) was added to the reaction solution at -70°C and stirred for 2 hours. After the completion of the reaction monitored by TLC, the reaction was quenched with ammonium chloride aqueous solution, extracted with ethyl acetate, and the organic layer was spin-dried and purified by a flash silica gel column to obtain 11.01 g of a solid with a yield of 94.42%.
[0033] Detected by mass spectrometry, ESI / MS: m / z=322.3[MH] + , the solid was determined to be 4-(6-trimethylacetamido-3-pyridyl)-4-hydroxy-N-tert-butoxycarbonylpiperidine.
[0034] ...
Embodiment 2
[0042] The first step: the synthesis of 4-(6-trimethylacetamido-3-pyridyl)-4-hydroxy-N-tert-butoxycarbonylpiperidine
[0043]At -65°C, slowly add n-butyllithium (1.94L, 4.85mol) in n-hexane solution (2.5N) into N-(5-bromo-pyridine-2-)-2,2-dimethylpropanamide (500 g, 1.94 mol) in tetrahydrofuran (1 L), the reaction mixture was stirred at -65°C to -70°C for 1 hour. N-tert-butoxycarbonyl-4-piperidone (465 g, 2.33 mol) in tetrahydrofuran (2 L) was added to the reaction solution at -65°C and stirred for 2 hours. After the completion of the reaction monitored by TLC, the reaction was quenched with ammonium chloride aqueous solution, extracted with ethyl acetate, and the organic layer was spin-dried and purified by a flash silica gel column to obtain 704.6 g of solid, with a yield of 96.0%.
[0044] Detected by mass spectrometry, ESI / MS: m / z=322.3[MH] + , the solid was determined to be 4-(6-trimethylacetamido-3-pyridyl)-4-hydroxy-N-tert-butoxycarbonylpiperidine.
[0045] The secon...
Embodiment 3
[0053] The first step: the synthesis of 4-(6-trimethylacetamido-3-pyridyl)-4-hydroxy-N-tert-butoxycarbonylpiperidine
[0054] At -78°C, slowly add n-butyl lithium (46.6L, 116.67mol) in n-hexane solution (2.5N) into N-(5-bromo-pyridine-2-)-2,2-dimethylpropanamide (10 kg, 38.89 mol) in tetrahydrofuran (20 L), the reaction mixture was stirred at -78°C to -70°C for 1 hour. N-tert-butoxycarbonyl-4-piperidone (7.75kg, 38.89mol) in tetrahydrofuran (12L) was added to the reaction solution at -78°C and stirred for 2 hours. After the completion of the reaction monitored by TLC, the reaction was quenched with ammonium chloride aqueous solution, extracted with ethyl acetate, and the organic layer was spin-dried and purified by a flash silica gel column to obtain 13.79 kg of solid with a yield of 93.9%.
[0055] Detected by mass spectrometry, ESI / MS: m / z=322.3[MH] + , the solid was determined to be 4-(6-trimethylacetamido-3-pyridyl)-4-hydroxy-N-tert-butoxycarbonylpiperidine.
[0056] Th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com