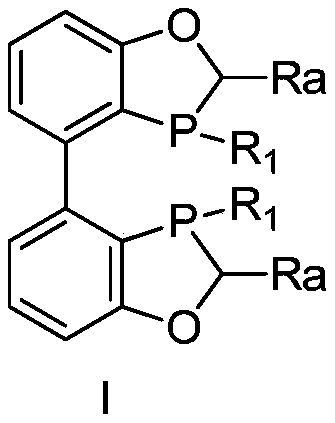
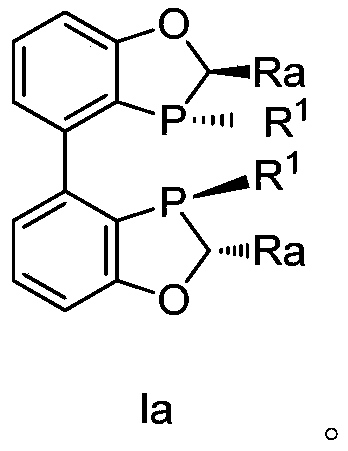
A kind of biaryl bisphosphine ligand, its preparation method and application
A technology of bisphosphine ligands and biaryls, applied in the field of bisaryl bisphosphine ligands, can solve the problems of low yield and optical purity of β-hydroxycarboxylates
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
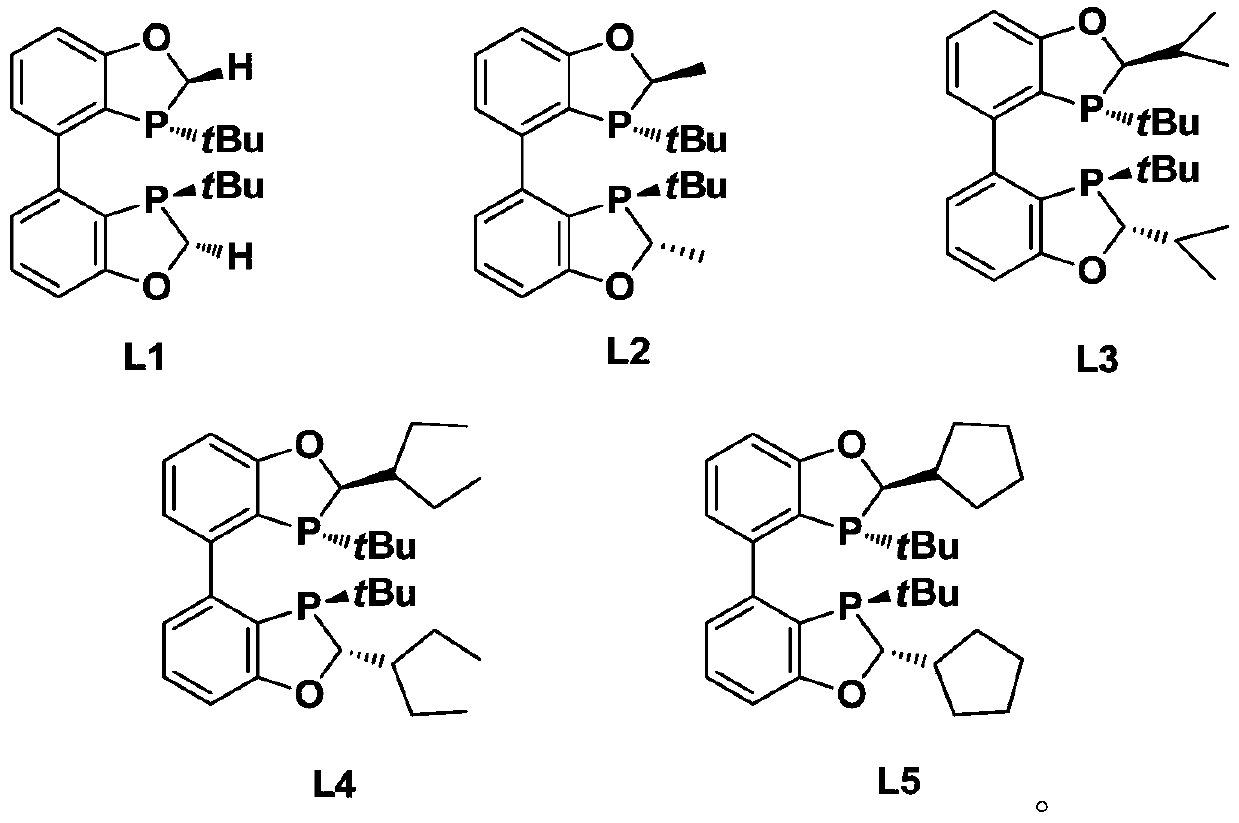
[0110] In this example, (3R,3'R)-3,3'-di-tert-butyl-2,2',3,3'-tetrahydro-4,4'-dibenzo[d][1,3 ] Oxygen, phosphorus-pentyl conjugate (L1) as an example to describe the preparation method of the chiral bisphosphine ligand of the present invention (the reaction scheme is as follows):
[0111]
[0112] 1. Preparation of (S)-3-tert-butyl-3-oxo-2H-benzo[d][1,3]oxy,phospho-pentyl-4-trifluoromethanesulfonate (1)
[0113] Prepared according to known literature methods, Org.Lett.2010,12,176.
[0114] 2. Preparation of (S)-(3-(tert-butyl)-3-oxo-2H-benzo[d][1,3]oxy,phospho-4-)carbamate (a)
[0115] Under nitrogen protection, (S)-3-tert-butyl-3-oxo-2H-benzo[d][1,3]oxy,phospho-pentyl-4-trifluoromethanesulfonate (1 , 5.5g, 15.4mol, 1.0 equivalent), cesium fluoride (3.3g, 21.5mmol, 1.4 equivalent) and tert-butyl carbamate (3.6g, 30.7mmol, 2.0 equivalent) were mixed and dissolved in 77mL tetrahydrofuran, and then three (Dibenzylideneacetone)dipalladium (699 mg, 0.76 mmol, 0.05 equiv) and ...
Embodiment 2
[0150] In this example, (2R,2'R,3R,3'R)-3,3'-di-tert-butyl-2,2'-dimethyl-2,2',3,3'-tetrahydro- 4,4'-Dibenzo[d][1,3]oxo,phosphorus-pentyl conjugate (L2) is used as an example to describe the preparation method of the chiral bisphosphine ligand of the present invention in detail (the reaction scheme is as follows):
[0151]
[0152] 1. (2R,2'R,3S,3'S)-3,3'-di-tert-butyl-2,2'-dimethyl-2H,2'H-[4,4'-dibenzo[d Preparation of ][1,3]oxo,phosphorus-pentaconjugate]3,3'-dioxo(3b)
[0153] Under the protection of nitrogen, 3a (0.2g, 0.48mmol, 1 equivalent) was dissolved in 10mL of tetrahydrofuran, the reaction solution was lowered to -78°C with a dry ice / acetone bath, and lithium diisopropylamide (1.2mL, 2.0 M in n-hexane / tetrahydrofuran, 2.39 mmol, 5 equivalents). Keep stirring at -78°C for 2 hours, then add iodomethane (0.15mmol, 2.39mmol, 5 equivalents), keep stirring at -78°C for 20 minutes, slowly return to room temperature, and react overnight. 10 mL of saturated ammonium chlo...
Embodiment 3
[0167] Referring to the preparation method of Example 2, the following chiral bisphosphine ligands L3 were prepared respectively
[0168]
[0169] 1. (2R,2'R,3S,3'S)-3,3'-di-tert-butyl-2,2'-diisopropyl-2H,2'H-[4,4'-dibenzo[ d] Preparation of [1,3]oxo,phosphorus-pentaconjugate]3,3'-dioxo (3c)
[0170] Under the protection of nitrogen, 3a (300mg, 0.72mmol, 1 equivalent) was dissolved in 15mL of tetrahydrofuran, the reaction solution was lowered to -78°C with a dry ice / acetone bath, and lithium diisopropylamide (1.8mL, 2.0M in n-hexane / tetrahydrofuran, 3.6 mmol, 5 equivalents). Keep stirring at -78°C for 2 hours, then add isopropyl iodide (0.36mmol, 3.59mmol, 5 equivalents), keep stirring at -78°C for 20 minutes, slowly return to room temperature, and react overnight. 10 mL of saturated ammonium chloride solution and 10 mL of dichloromethane were added to the reaction solution. The organic phase was separated, and the aqueous phase was further extracted with (10 mL×2) dichl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com