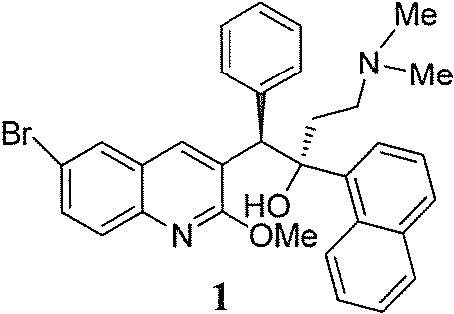
A method for separating bedaquiline diastereomer a
A technology of diastereoisomers and bedaquiline, applied in the field of separation of bedaquiline diastereomer A, can solve the problem of low purity of diastereomer A and difficult control of reaction conditions , Inability to purify and separate products, etc., to achieve high purity and yield, high purity, and the effect of easy raw material residue
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
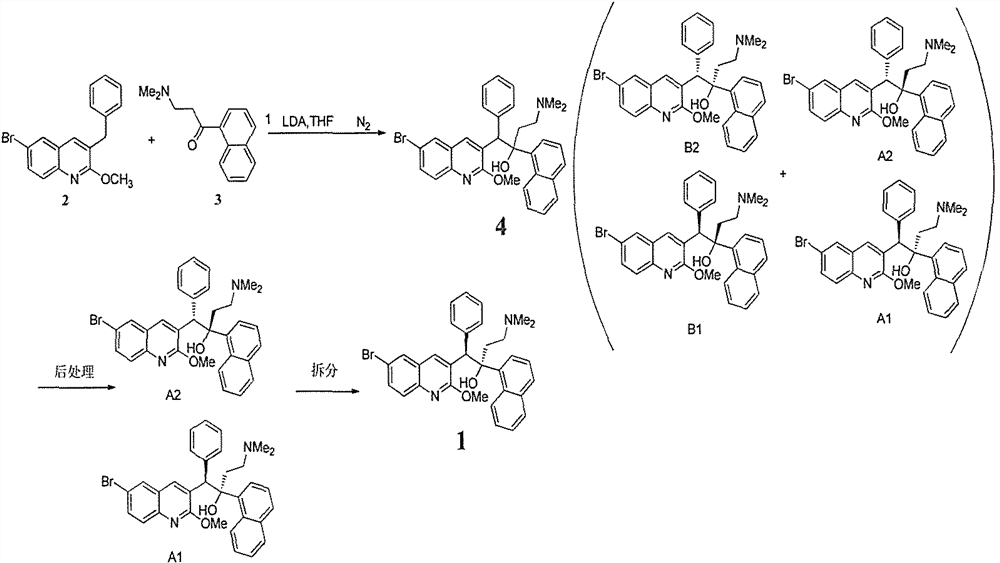
[0043] Reaction materials 3-benzyl-6-bromo-2-methoxyquinoline (10g) and 3-dimethylamino-1-(naphthalene-5-yl)acetone (10g), in tetrahydrofuran (80ml) with LDA (20g) reaction, one-step reaction obtains the bedaquiline reaction liquid of racemization. The conversion of the reaction was 56% as analyzed by HPLC. After the reaction was quenched, n-heptane (40ml) was added to the reaction solution, and the unnecessary diastereoisomer B was precipitated at 0°C in an ice-water bath, and the non-enantiomer B was removed by filtration. The obtained filtrate was washed with 50% acetic acid aqueous solution to remove the raw material 3-dimethylamino-1-(naphthalene-5-yl) acetone, and 15% hydrochloric acid aqueous solution was added to the organic layer to stir, so that the product was formed into a salt in the aqueous layer. Precipitate in. After filtration, the filtrate was separated into layers, at which point the product was transferred to the aqueous layer, and the starting material 3...
Embodiment 2
[0048] Starting material 3-benzyl-6-bromo-2-methoxyquinoline (10g) and 3-dimethylamino-1-(naphthalene-5-yl)acetone (10g), in tetrahydrofuran (80ml) and LDA ( 20g) reaction, one-step reaction obtains the bedaquiline reaction liquid of racemization. The conversion of the reaction was 65% as analyzed by HPLC. After the reaction was quenched, diisopropyl ether (160ml) was added to the reaction solution, and the unnecessary diastereoisomer B was precipitated in an ice-water bath at 5°C, and the non-enantiomer B was removed by filtration. The obtained filtrate was washed with 10% formic acid aqueous solution to remove the raw material 3-dimethylamino-1-(naphthalene-5-yl) acetone, and 5% sulfuric acid aqueous solution was added to the organic layer and stirred to make the product salted in the aqueous layer Precipitate in. After filtration, the filtrate was separated into layers. At this time, the product was transferred to the water layer, and the raw material 3-benzyl-6-bromo-2-m...
Embodiment 3
[0053] Starting material 3-benzyl-6-bromo-2-methoxyquinoline (10g) and 3-dimethylamino-1-(naphthalene-5-yl)acetone (10g), in tetrahydrofuran (80ml) and LDA ( 20g) reaction, one-step reaction obtains the bedaquiline reaction liquid of racemization. The conversion of the reaction was 75% by HPLC analysis. After the reaction was quenched, diisopropyl ether (400ml) was added to the reaction solution, and the unnecessary diastereoisomer B was precipitated in an ice-water bath at 2°C, and the non-enantiomer B was removed by filtration. The obtained filtrate was washed with 60% propionic acid aqueous solution to remove the raw material 3-dimethylamino-1-(naphthalene-5-yl) acetone, and 40% methanesulfonic acid aqueous solution was added to the organic layer and stirred to make the product into a salt Precipitate in the water layer. After filtration, the filtrate was separated into layers, at which point the product was transferred to the aqueous layer, and the starting material 3-be...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com