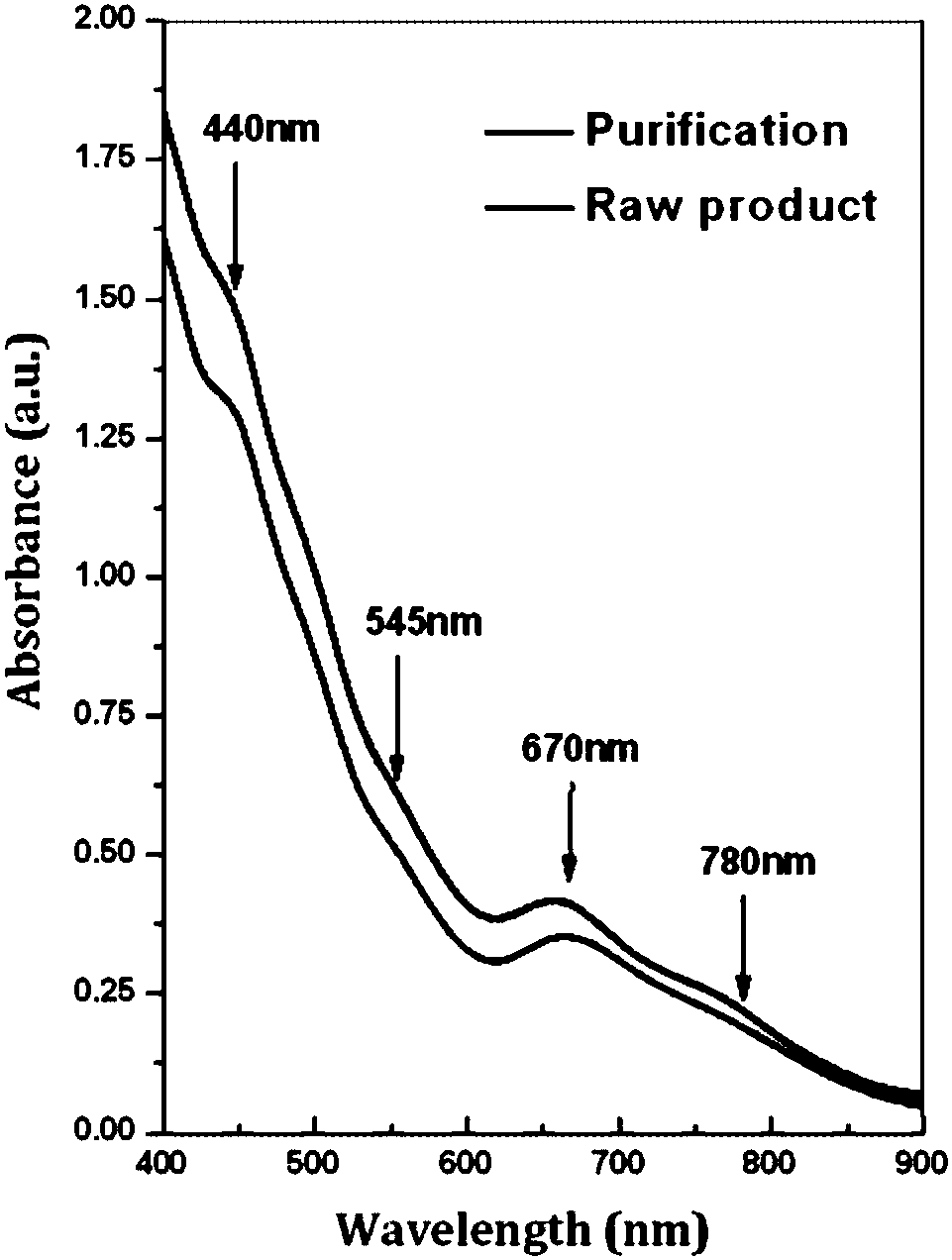
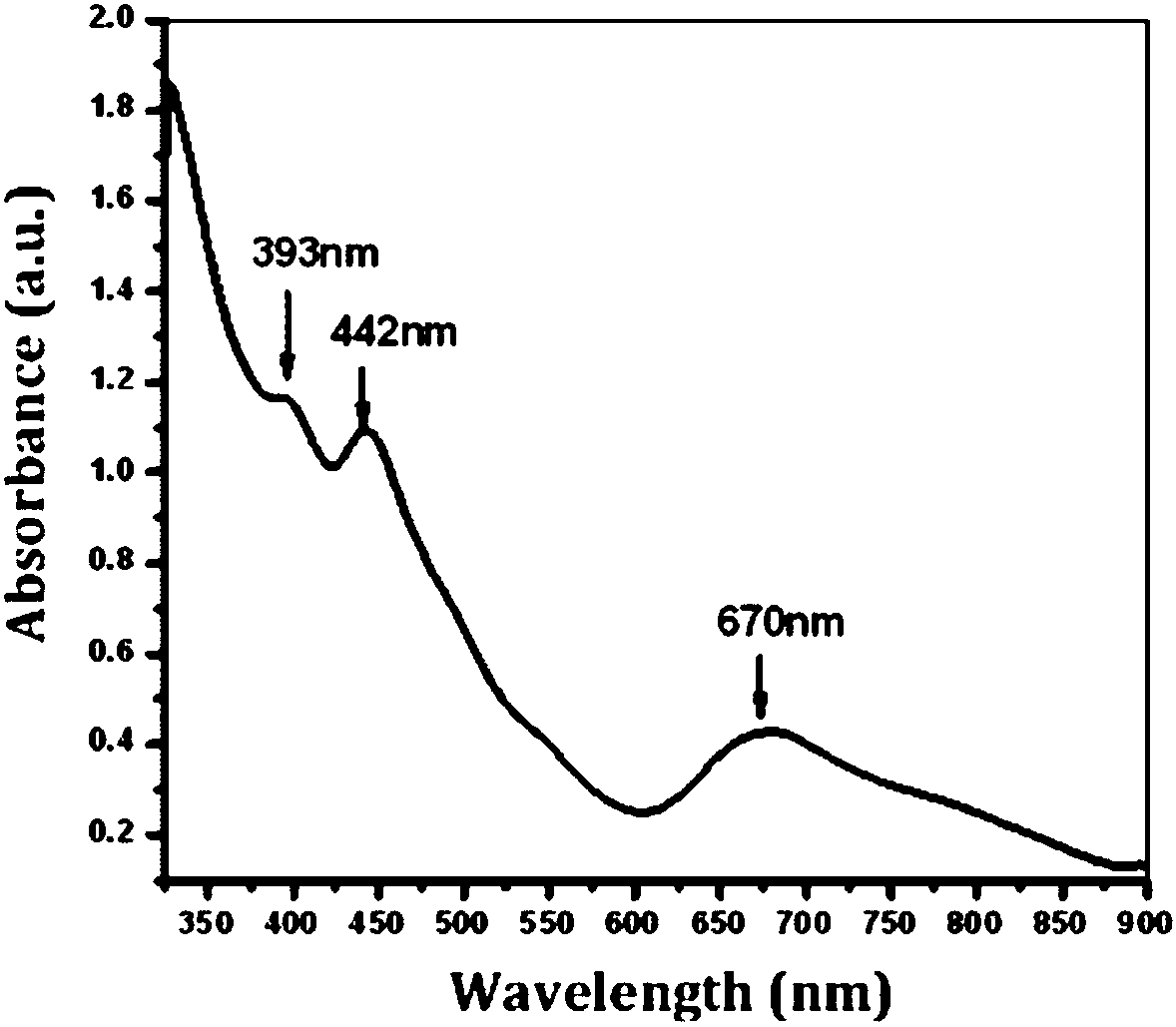
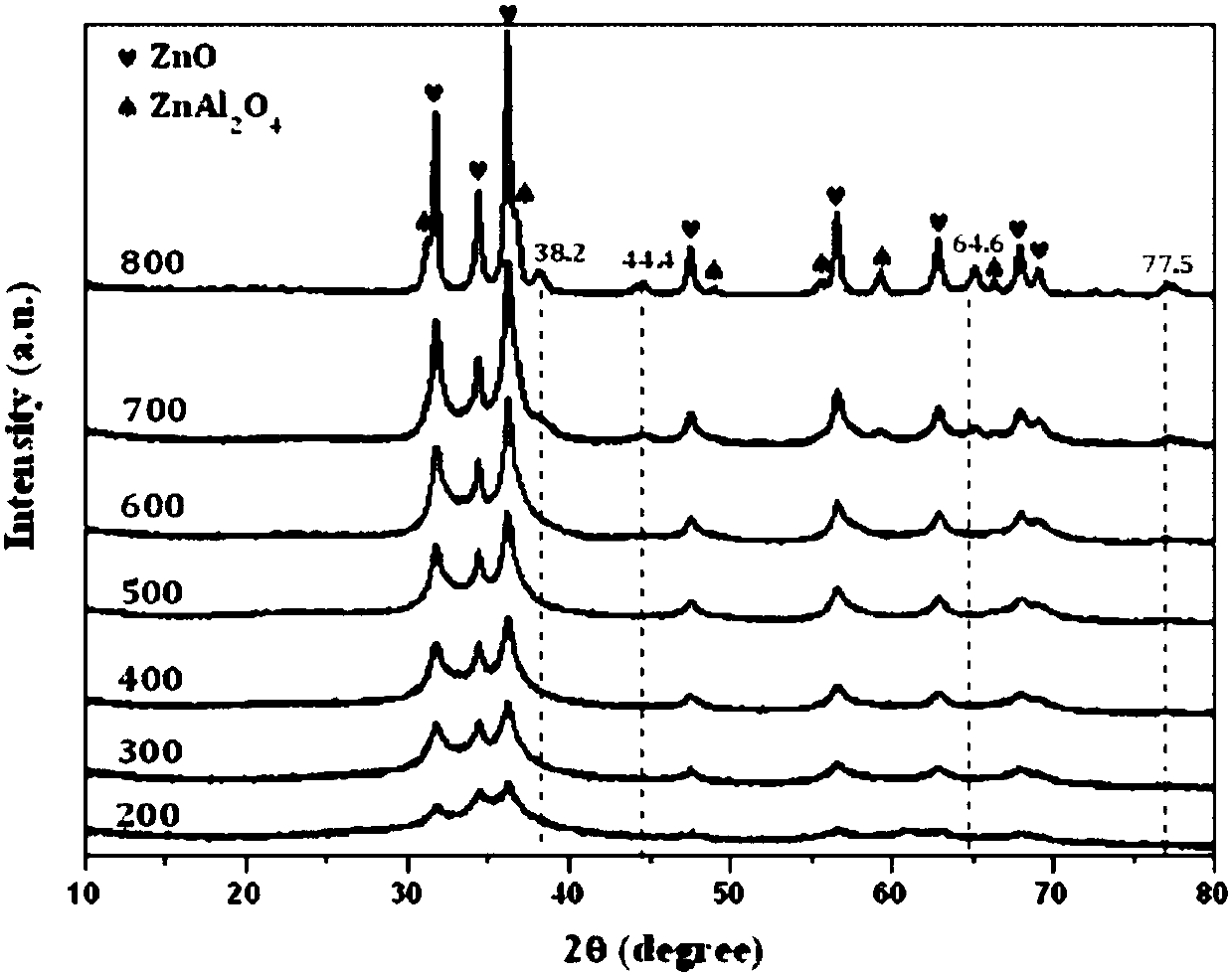
Gold cluster catalyst for efficiently catalyzing selective hydrogenation of unsaturated aldehyde ketone to produce unsaturated alcohol
A selective hydrogenation and unsaturation technology, applied in metal/metal oxide/metal hydroxide catalysts, physical/chemical process catalysts, chemical instruments and methods, etc., can solve problems such as inability to guarantee high selectivity of unsaturated alcohols , to achieve good catalytic performance, good selectivity and low price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Preparation of aqueous phase Au 25 atomic cluster
[0026] Prepare chloroauric acid solution, cysteine solution, 1M NaOH solution and NaBH in advance 4 (0.2M NaOH) solution. Add 5.00 mL HAuCl to a 1000 mL round bottom flask 4 Solution (20.89g Au / L), 200mL ultrapure water and 150mL cysteine solution, stirred for 40min, and it was observed that the color of the solution changed from light yellow to dark yellow to milky white. Then measure 30mL of 1M NaOH solution and add it into the flask quickly, and the solution becomes clear and transparent. Freshly prepared NaBH was then 4 (0.2M NaOH) solution 10mL was added in the flask, the solution turned brownish red, stirred and reacted at room temperature for 3h, the color of the solution gradually deepened and finally turned brownish black. Cysteine, NaOH, NaBH in the above process 4 The molar ratios to Au are 1.5, 30, and 5, respectively. The obtained product was centrifuged and washed with a solution of ethanol / w...
Embodiment 2
[0028] Preparation of organic phase Au 25 atomic cluster
[0029] Use a pipette to measure 3.67mL (0.32mmol, 17.18gAu / L) of chloroauric acid solution, dissolve it in 20mL ultrapure water, place it in a 250mL round bottom flask, add tetraoctylammonium bromide (0.384 mmol, 210 mg) in 50 mL of toluene, stirred at 500 rpm for 30 min, and when the gold in the aqueous phase was completely transferred to the organic phase, the colorless aqueous layer was removed with a syringe. Then the stirring speed was reduced to 50rpm, and 400uL dodecanethiol was added to the organic phase, and the color of the solution was observed to change from orange-red to white within 5min. After reacting for 30 minutes, 50 mL of toluene solution was added to dilute, and the stirring speed was increased to 800 rpm. At the same time, 25 mL (3.2 mmol, 121 mg) of sodium borohydride aqueous solution was added under stirring conditions, and then the reaction was stirred at room temperature for 22 h. And the so...
Embodiment 3
[0031] Preparation of hydrotalcite carrier
[0032] Take Zn(NO 3 ) 2 .6H 2 O(or Mg(NO 3 ) 2 .6H 2 O, or Ni(NO 3 ) 2 .6H 2 O, or Co(NO 3 ) 2 .6H 2 O) 0.21mol, Al(NO 3 ) 3 9H 2 Add 0.07mol of O into a 1000mL beaker, then add 200mL of deionized water and stir to dissolve to form A solution. Take NaOH 0.438mol, Na 2 CO 3 Add 0.113mol into another 500mL beaker, add 200mL deionized water and stir to dissolve to form B solution. Under stirring in a water bath at 75°C, slowly drop the A solution into the B solution with a constant flow pump, and then stir and age at this temperature for 24 hours. The resulting suspension was filtered and washed with a large amount of deionized water, and finally dried in an oven at 60°C overnight. After the dried sample was ground with an agate mortar, the 120-mesh powder was sieved with a mesh sieve, bottled and stored in a desiccator. Other hydrotalcite supports with different proportions were prepared by controlling the amount o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com