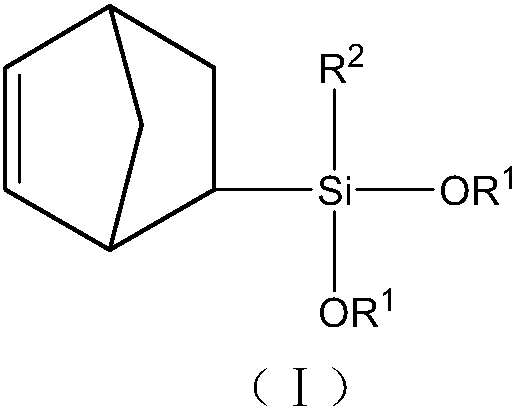
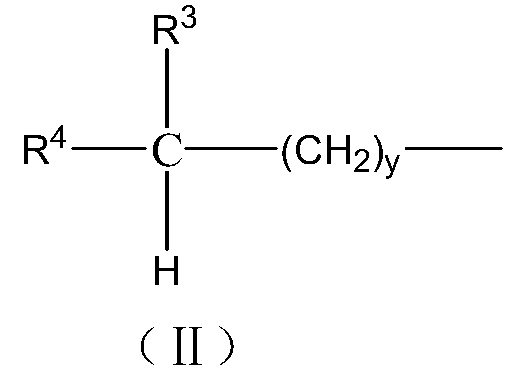
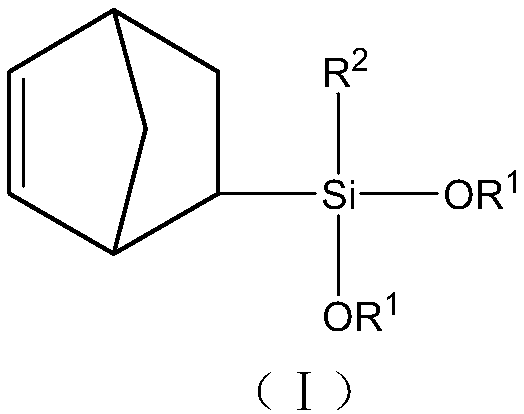
Norbornene organo-dialkoxysilane compound and preparation method thereof
A technology of base dialkoxy silane and alkenyl trialkoxy silane, applied in norbornenyl organo dialkoxy silane compound and preparation of the compound, in the field of external electron donor, achieving high activity and cost Advantages, the effect of good comprehensive performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] A Preparation of norbornenyltrimethoxysilane:
[0046] In a 1L stainless steel autoclave equipped with a mechanical stirrer, a thermometer, a nitrogen gas inlet, a feed port, and a liquid sampling port, add 153.8 g of vinyltrimethoxysilane, 80 g of dicyclopentene, and 300 g of methanol, and under stirring, the reaction mass It was heated up to 180°C and kept for 5 hours for reaction. Samples were taken for gas chromatographic analysis to monitor the extent of the reaction. After the reaction is completed, the solvent, unreacted raw materials such as cyclopentadiene, dicyclopentadiene, vinyltrimethoxysilane, etc. are distilled off at normal pressure, and then collected by distillation at -0.098MPa under reduced pressure at 135-156°C The distillate product is 230 grams, and the yield is 75% (based on vinyltrimethoxysilane). The reaction process can be represented by reaction formula 1:
[0047] Reaction 1
[0048]
[0049] B preparation of norbornenyltrimethoxysila...
Embodiment 2
[0052] Preparation of norbornenylcyclopentyldimethoxysilane
[0053] Replace the air with nitrogen in a 500mL dry four-necked flask equipped with an electric stirrer, a thermometer, a reflux condenser, and a constant pressure dropping funnel, and drop 12.1g (purity 99%, 0.5mol) into the reaction flask under a nitrogen atmosphere. Magnesium powder less than 100 meshes, 2.7g (purity 99%, 0.026mol) chlorocyclopentane, 20ml methyl tert-butyl ether. Add a mixed solution of 51.2g (0.49mol) cyclopentane chloride and 100ml methyl tert-butyl ether into the constant pressure dropping funnel. Add a small amount of initiator iodine to the contents of the reaction vial. After the reaction is initiated, stir. When the exothermic reaction is stable, add the mixture in the dropping funnel dropwise to the reaction flask within 30-60 minutes, and control the reaction temperature so that the solvent is in a reflux state; the mixture of chlorocyclopentane and methyl tert-butyl ether After comp...
Embodiment 3
[0060] Preparation of norbornyl isobutyldimethoxysilane
[0061] According to the conditions of Example 2, the chlorocyclopentane was replaced by an equimolar chloroisobutane; after the reaction was completed, the solvent was recovered by filtration, washing, normal pressure and the previous fraction was evaporated, and finally the vacuum distillation was collected at 118-120°C / -0.098MPa fraction. 80 g of norbornyl isobutyldimethoxysilane was obtained with a purity of 98% and a yield of 72% (calculated as norbornyl trimethoxysilane). The reaction process is represented by reaction formula 3:
[0062] Reaction 3
[0063]
[0064] The hydrogen spectrum result of norbornenyl isobutyl dimethoxysilane compound is:
[0065] 1 H NMR (600MHz, CDCl 3 )δ6.28–5.84(m,2H),3.68–3.42(m,6H),3.13–2.80(m,2H),2.31–1.07(m,6H),0.99(ddd,J=18.1,7.8,3.2 Hz,6H), 0.72–0.52(m,2H).
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size (mesh) | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com