Method for determining content of impurities in linagliptin raw material
A technology of impurity content and API, applied in the field of biomedicine, can solve the problems affecting the quality of linagliptin products and the efficacy of linagliptin, and achieve the effects of quality control, good separation, and real measurement.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] In this example, the inventors introduced in detail the development process of the method for determining the impurity content in linagliptin bulk drug.
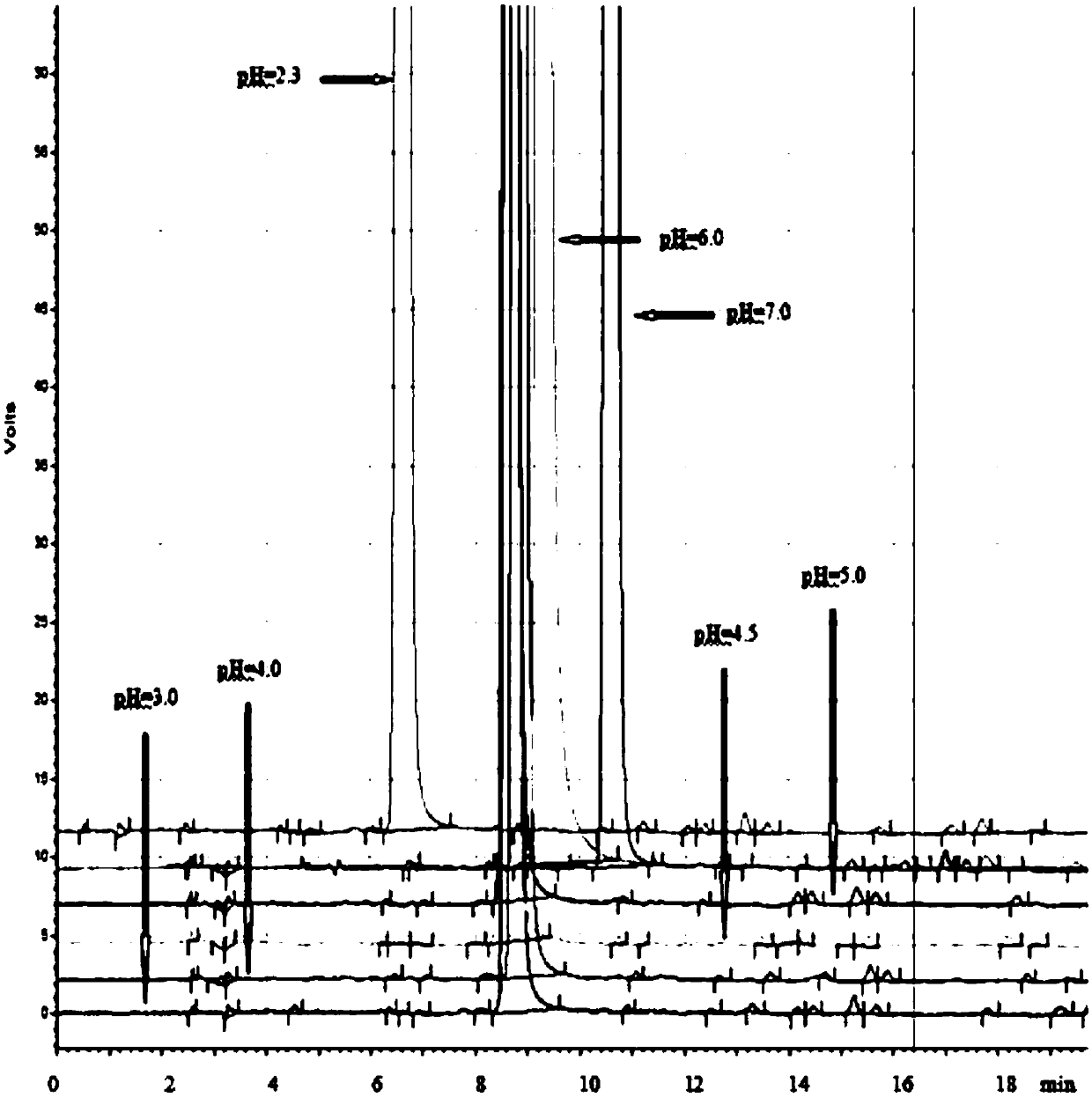
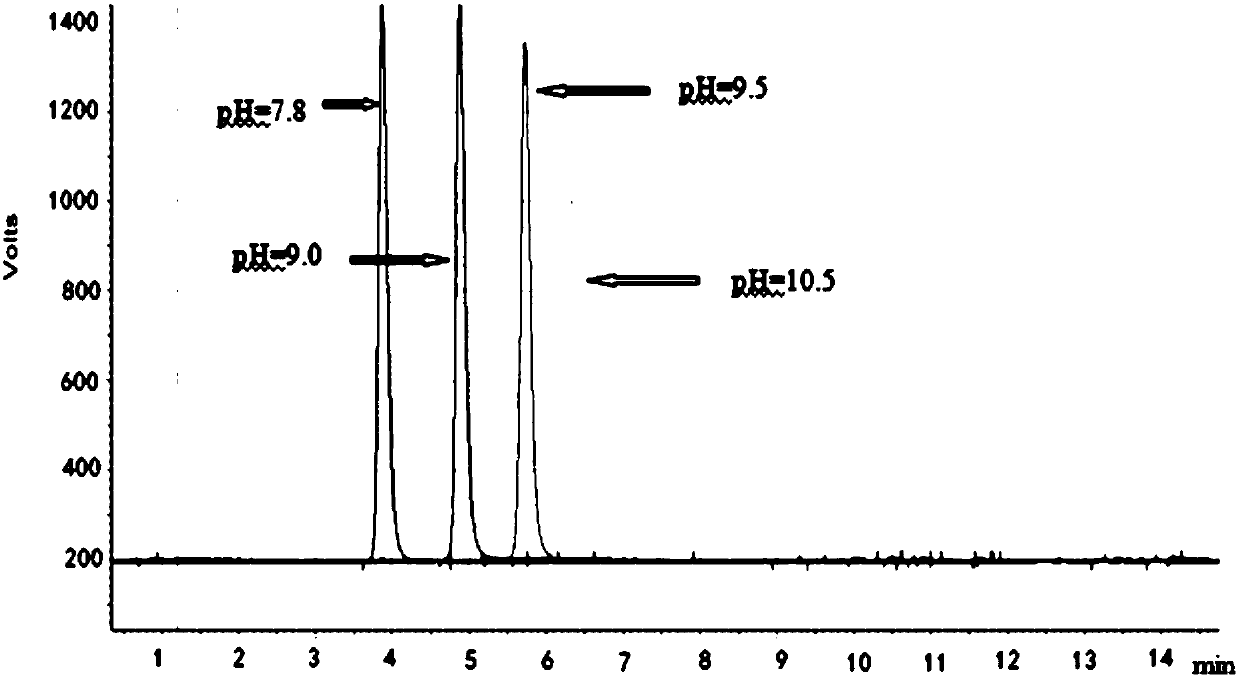
[0058] 1.1 Determination of buffer salt type and pH value
[0059] Linagliptin contains a primary amino group, and the CAD software predicts that the pKa of its conjugate acid is as high as 10.0, which is relatively strong; Linagliptin also contains multiple basic groups, all of which are connected with more complex groups. It is difficult to judge the acidity and alkalinity. It is necessary to select a buffer with an appropriate pH value to ensure that the compound to be separated exists in a single form during the separation process, so as to achieve the separation effect of a single peak, a sharp peak shape, and a reproducible retention time. Therefore, in the initial stage of method development, the inventors investigated the separation effect of linagliptin and corresponding impurities under the same gradient and ...
Embodiment 2
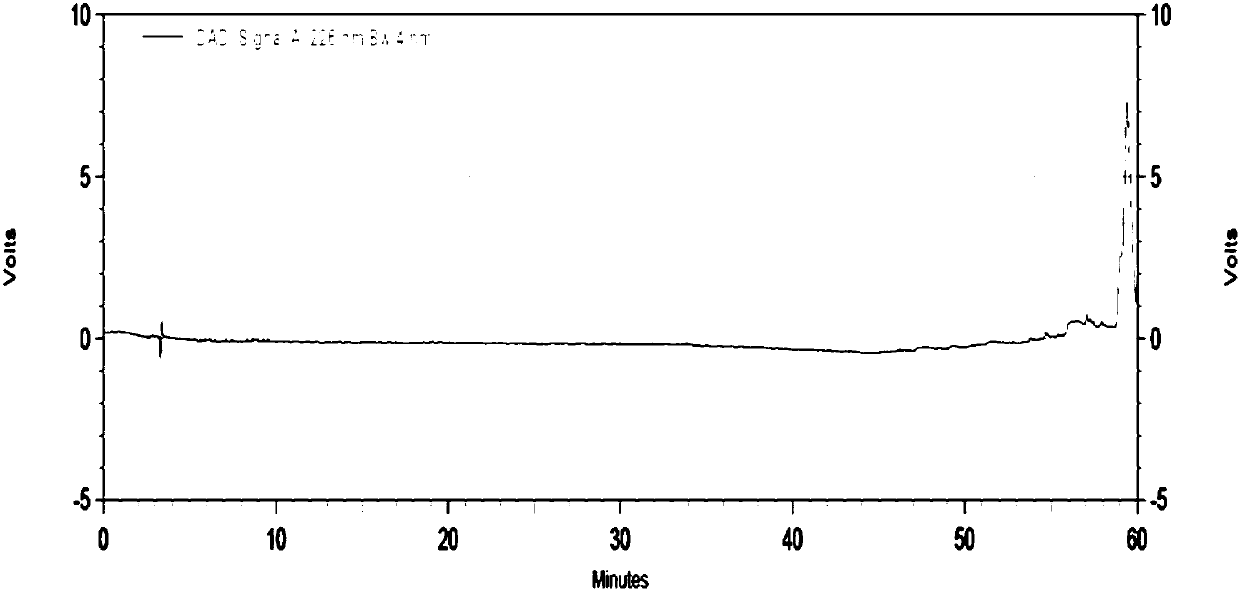
[0086] In this example, the inventor investigated the system applicability and sensitivity of the method for determining the impurity content in Linagliptin bulk drug determined in Example 1, and introduced in detail how to use the chromatographic conditions determined in Example 1 The obtained chromatograms were used to calculate the impurity content.
[0087] 2.1 Preparation of relevant solutions
[0088] The diluent (blank solution) is: acetonitrile: phase A = 1:4 mixed solution;
[0089] Test solution: Take about 20 mg of the test product, weigh it accurately, put it in a 100 mL volumetric flask, dissolve it with an appropriate amount of diluent, sonicate and dilute to the mark, shake well, and prepare 2 parts in parallel;
[0090] System suitability solution: Take about 4 mg of impurity A reference substance, weigh it into a 100mL volumetric flask, accurately pipette 5mL of the test solution 1 into the same 100mL volumetric flask, add diluent to dissolve and dilute to th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com