Novel biodegradable controlled-release preparation of Pin1 inhibitor ATRA, and preparing method and application thereof
A technology of biodegradable and controlled-release preparations, applied in anti-inflammatory agents, pharmaceutical formulations, microcapsules, etc., can solve the problems of costing money, destroying the carrier structure, and time-consuming, and achieve high encapsulation efficiency and yield, and biocompatibility Good sex, enhanced inhibitory effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
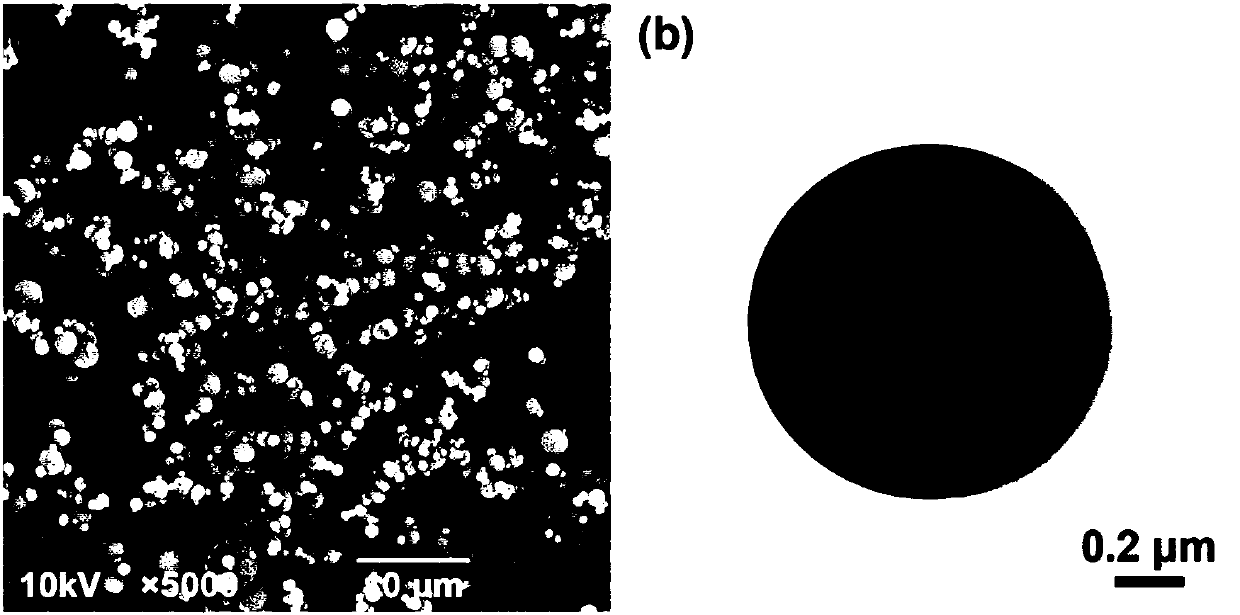
[0048] Add ATRA and PLLA to the organic solvent dichloromethane, and obtain a homogeneous oil phase by stirring, wherein the concentration of PLLA is 1% (% is mass volume percentage, % in the embodiments of the present invention refers to kg / L), ATRA and PLLA The mass ratio is 3%; the oil phase is pumped (flow rate is 1.0 mL / min) into the reactor, under the condition of supercritical carbon dioxide (pressure is 7.6 MPa; temperature is 45°C, carbon dioxide flow rate is 40 g / min ), to obtain ATRA-loaded PLLA microspheres, and then continue to dry the microspheres with supercritical carbon dioxide for 30 min to fully remove dichloromethane. Blank PLLA microspheres and FITC-loaded PLLA microspheres were prepared under the same experimental conditions. Scanning electron microscopy (SEM) results showed that ATRA-PLLA microspheres had good sphericity and uniform particle size. Transmission electron microscopy (TEM) results showed that ATRA-PLLA microspheres were solid microspheres. ...
Embodiment 2
[0051] Weigh 4 mg of ATRA-PLLA microspheres, add them into 4 mL of dichloromethane, and dissolve them fully with magnetic stirring, and measure the absorbance of the solution at 360 nm with a microplate reader. The blank PLLA microspheres were dissolved in dichloromethane as a background control. The ATRA standard curve was established with the ATRA standard substance, and the concentration of ATRA in the dichloromethane solution was calculated. In addition, 4 mg of ATRA-PLLA microspheres were weighed and added to 4 mL of 75% ethanol to elute the loosely adsorbed or bound ATRA on the surface of the microspheres, and then filtered with a 0.22 μm filter membrane and read at 360 nm with a microplate reader. Measure the absorbance of the ethanol solution. The concentration of ATRA in the ethanol solution was calculated according to the ATRA standard curve. The drug loading, encapsulation efficiency and yield were calculated according to the formula. Drug loading (%) = (weight o...
Embodiment 3
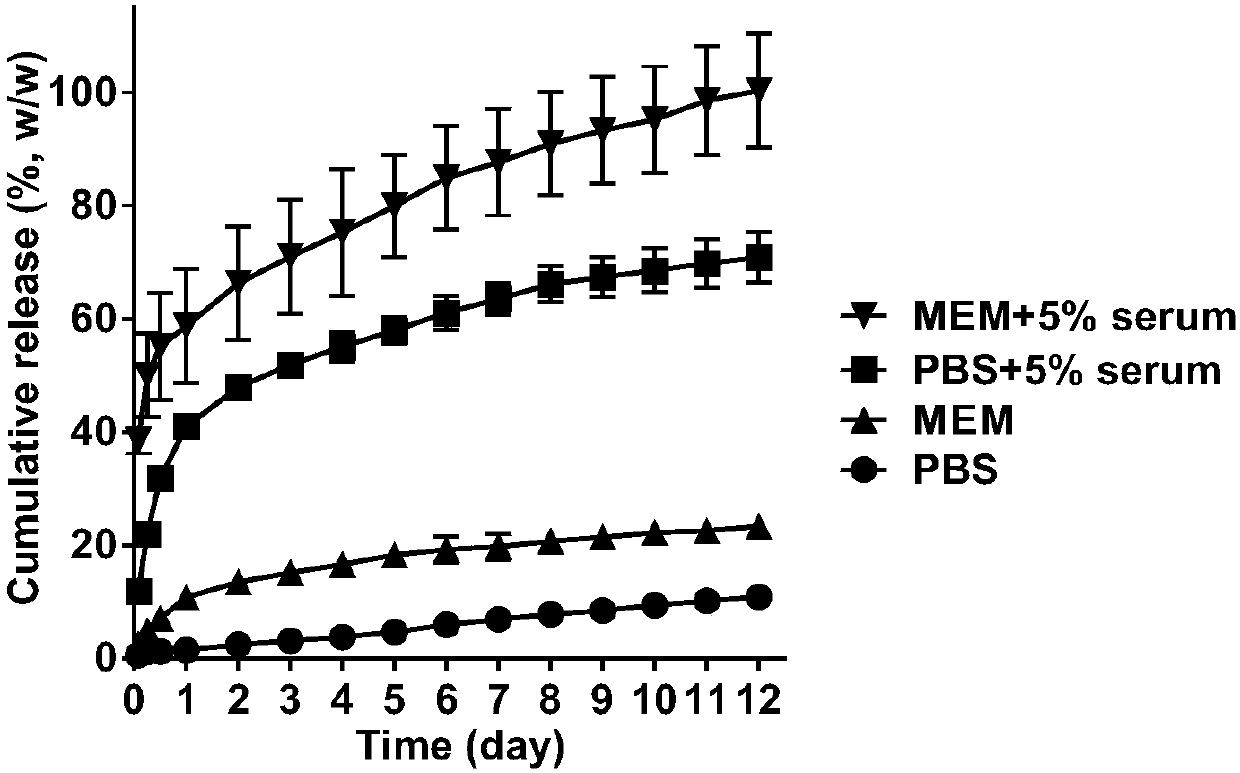
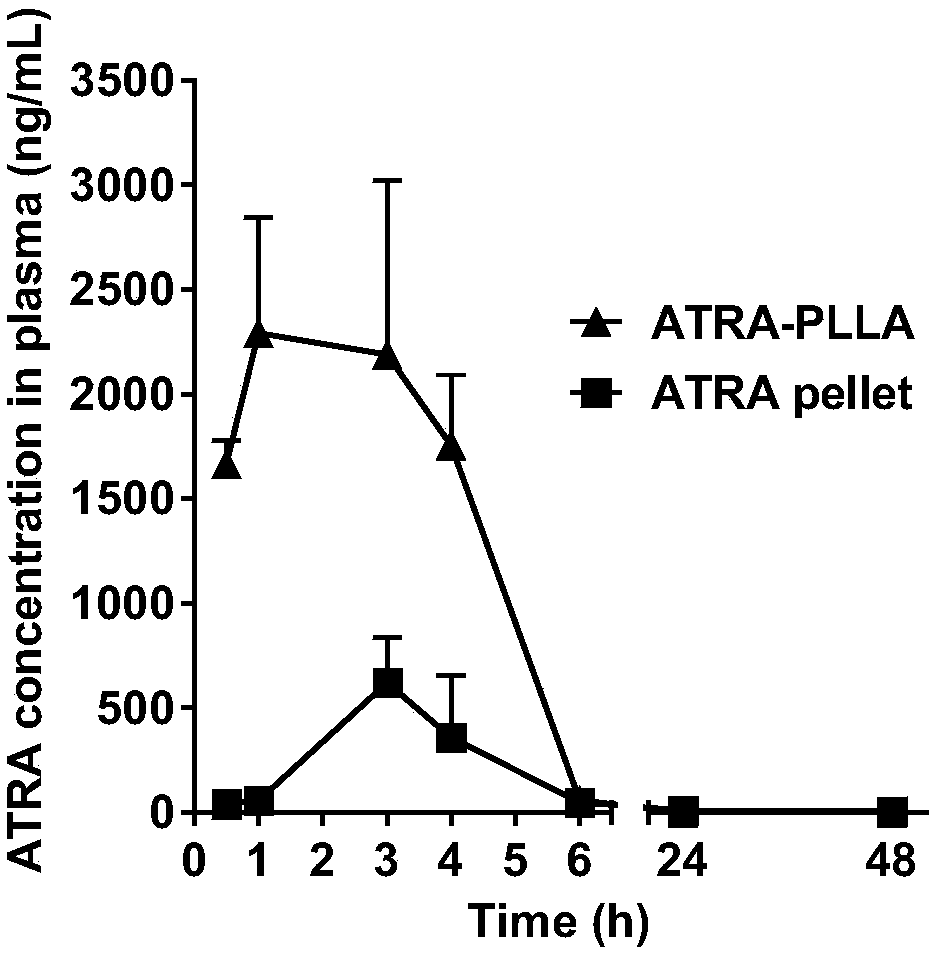
[0053] Weigh 2 mg of ATRA-PLLA microspheres, disperse them in 1 mL of PBS (pH7.4), PBS containing 5% serum, MEM medium, or MEM medium containing 5% serum, and place at 37°C , 100 rpm shaker, at different time points (2 h, 6 h, 12 h, 1 d, 2 d, 3 d, 4 d, 5 d, 6 d, 7 d, 8 d, 9 d, 10 d , 11 d, 12 d), take out the experimental samples, centrifuge at 10,000 rpm for 5 min, collect the supernatant, then resuspend the microspheres with the fresh above-mentioned release medium, and put them back in the shaker to continue the experiment. The concentration of ATRA in each supernatant was measured, and the cumulative release rate of ATRA was calculated. As a result, ATRA-PLLA microspheres could release ATRA for 12 days even in serum-containing MME medium; in serum-free medium solution, there was no burst release phenomenon of ATRA-PLLA microspheres. That is, ATRA-PLLA microspheres can effectively release ATRA in vitro.
[0054] figure 2 It is the in vitro release profile of ATRA-PLLA m...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com