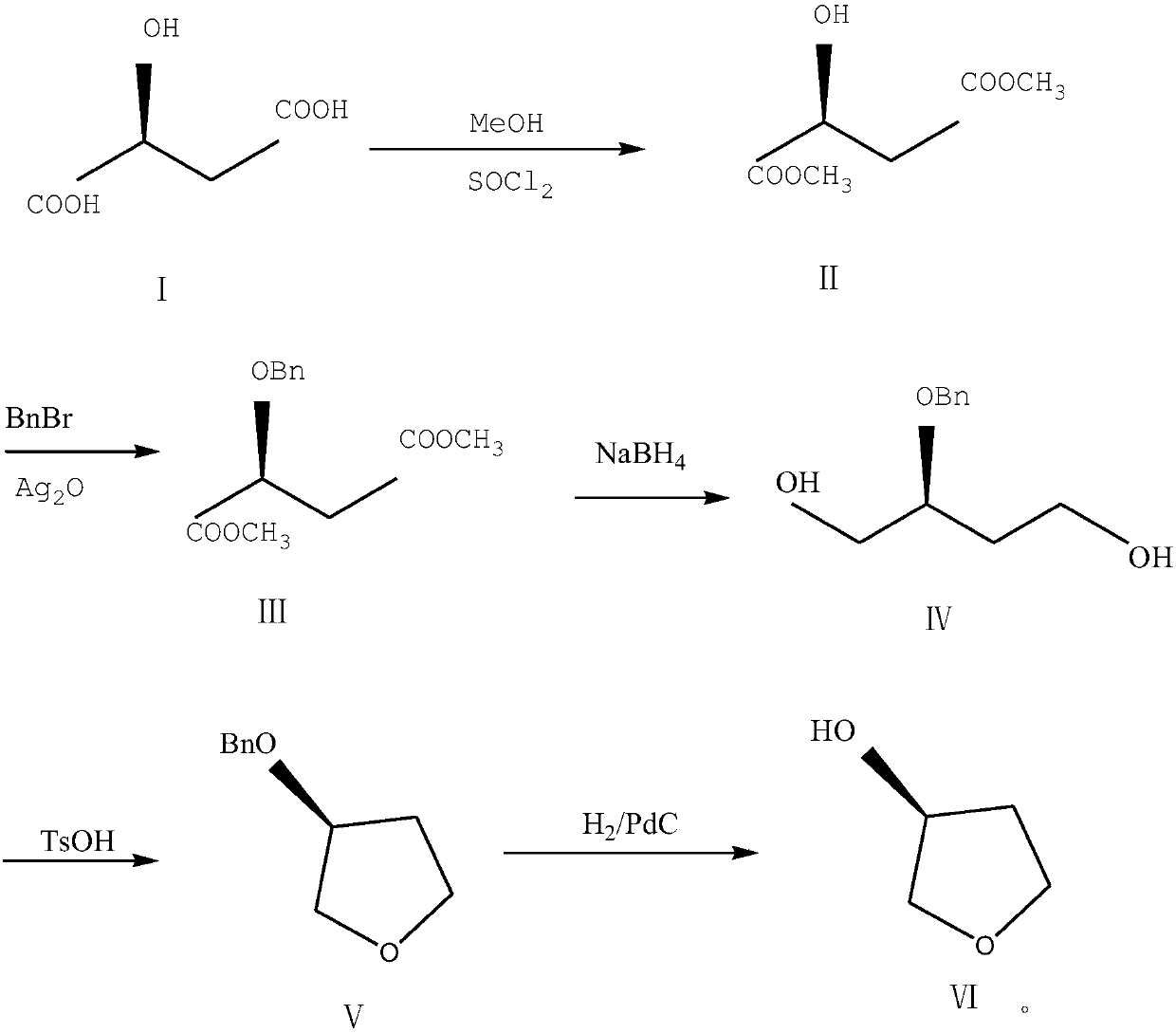
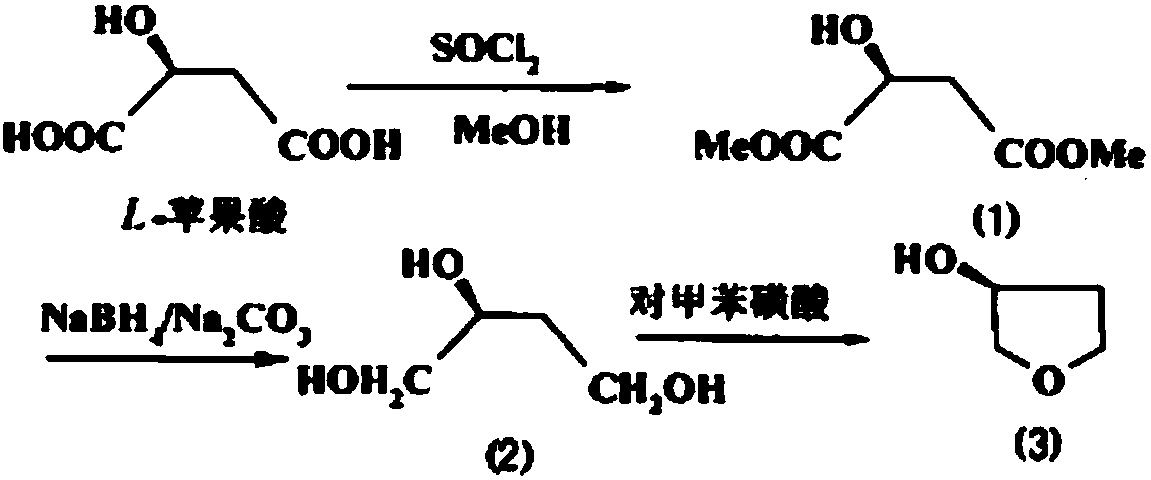
Preparation method of (S)-3-hydroxytetrahydrofuran
A technology of hydroxytetrahydrofuran and sodium borohydride, applied in the direction of organic chemistry, can solve the problems of increased product cost, impurities, high reaction temperature, etc., and achieve the effects of increased product yield, omitted rectification steps, and simple post-treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] 1) Add 280mL methanol, 168g (1.25mol) L-malic acid to a 1000ml reaction flask, reduce it to 0°C, add 202mL thionyl chloride dropwise to the temperature below 0°C, and then raise it to room temperature after dripping, and react with stirring for 24 hours; After the reaction is completed, the methanol is evaporated at 40-45°C.
[0040] 2) Add 215 g (1.25 mol) of benzyl bromide and 2 g of silver oxide to the obtained oil, stir and react at room temperature for 6 hours, and filter with suction.
[0041] 3) Add 600 mL of tetrahydrofuran and 44 g of sodium borohydride to the reaction flask, lower the temperature to 0°C, and control the temperature at about 0°C to drop the mother liquor obtained in the step. After dripping, the reaction was kept at 0°C for 18 hours. After the reaction, tetrahydrofuran was distilled off, the pH was adjusted to 2 with concentrated hydrochloric acid, 300 mL of ethyl acetate was added for extraction, and the mixture was concentrated under reduced pres...
Embodiment 2
[0045] 1) Add 260 mL of methanol and 168 g (1.25 mol) of L-malic acid to a 1000 ml reaction flask, reduce to 0°C, add 200 mL of thionyl chloride dropwise to control the temperature below 0°C, and then raise to room temperature after dropwise, and react with stirring for 27 hours. After the reaction is completed, the methanol is evaporated at 40-45°C.
[0046] 2) Add 215 g (1.25 mol) of benzyl bromide and 2 g of silver oxide (recovered) to the obtained oil, stir and react at room temperature for 5 hours, and filter with suction.
[0047] 3) Add 500 mL of tetrahydrofuran and 44 g of sodium borohydride to the reaction flask, lower the temperature to 0°C, and control the temperature at about 0°C to drop the mother liquor obtained in the step. After dripping, keep the reaction at 0°C for 20 hours. After the reaction, the tetrahydrofuran was distilled off, the pH was adjusted to 2 with concentrated hydrochloric acid, 280 mL of ethyl acetate was added for extraction, and the mixture was ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com