Preparation method and product of 3D cross-linked hyaluronate gel for radiotherapy protection
A technology of cross-linking hyaluronic acid and hyaluronate, which is applied to medical preparations containing active ingredients, pharmaceutical formulas, organic active ingredients, etc., to achieve the effects of good enzymolysis resistance, stable 3D structure, and reasonable reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Example 1 Preparation of cross-linked hyaluronic acid
[0033] Take 0.01 g of BDDE and mix with 5 mL of 1 wt% sodium hydroxide solution evenly, then add 1.0 g of sodium hyaluronate (3.5 million Da) and stir to dissolve evenly. The above reactants were sealed and left to react at 3 °C for 15 h, then at 50 °C for 3 h, and finally at 20 °C for 15 h. Cut the final reaction product into about 1 cm 3 The small cubes were placed in phosphate sodium chloride buffer and dialyzed, and the resulting gel block was granulated through a 60-mesh sieve to obtain a cross-linked hyaluronic acid gel with a content of 20 mg / mL. Autoclaved at 121°C for 8 min.
Embodiment 2
[0034] Example 2 Preparation of cross-linked hyaluronic acid
[0035] Except that the crosslinking agent is different (BDDE is replaced with carbodiimide), other reaction temperature and time are all the same as in Example 1. Finally, a cross-linked hyaluronic acid gel with a hyaluronic acid content of 20 mg / mL was prepared. Autoclaved at 121°C for 8 min.
Embodiment 3
[0042] Example 3 In vitro resistance to enzymatic hydrolysis of cross-linked hyaluronic acid
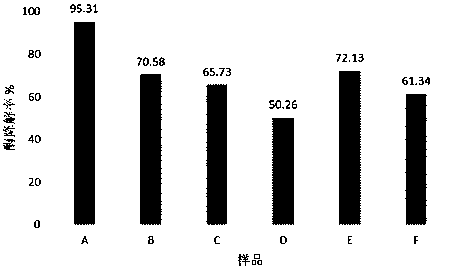
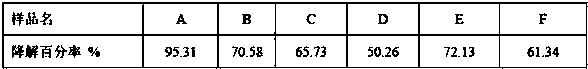
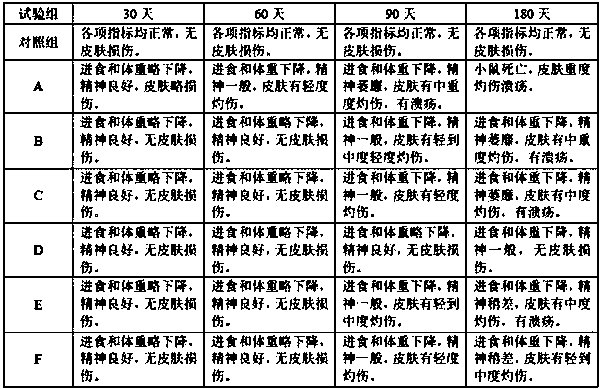
[0043] The gel obtained by one-step cross-linking in the comparative example is designated as gel A, the gel obtained by two-step cross-linking of "low temperature-high temperature" is designated as gel B, and the gel obtained by two-step cross-linking of "high temperature-low temperature" is designated as gel C; the hyaluronic acid gel obtained in Example 1 is marked as Gel D, the DVS cross-linked gel in Comparative Example 1 is marked as Gel E, and the carbodiimide cross-linked gel in Example 2 is marked as Gel F.
[0044] Weigh the appropriate amount of six samples of A, B, C, D, E, and F (the content of cross-linked hyaluronic acid is 0.5 g) into a vial, add 5 mL of 0.1 mol / L phosphate buffer (pH 7.0) and 5 mL of hyaluronidase solution (100 U / mL), mix well, place in a 37°C water bath for 24 h, and then boil at 100°C for 10 min to inactivate. Filter through a 0.45 μm microporous ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com