Preparation method and chemiluminescence analysis method of R6GHO
A chemiluminescence and hydrazide technology, which is applied in the field of chemiluminescence determination of nitrite, can solve the problems of low selectivity and limited measurement objects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
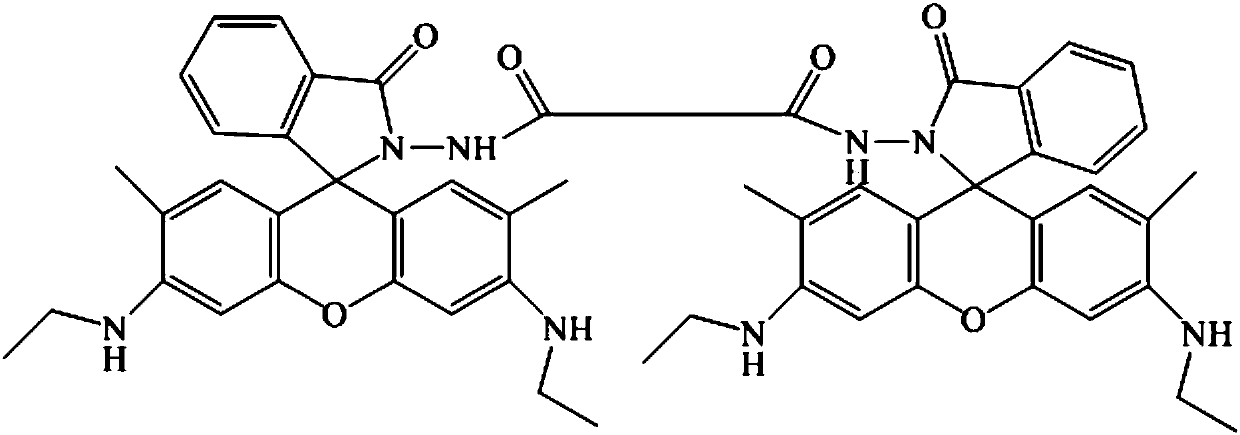
[0029] 1) In a 100mL three-necked flask, add 2.40g of rhodamine 6G and 60mL of hot absolute ethanol, cool slightly after the rhodamine 6G is completely dissolved, then add 8mL of 80% hydrazine hydrate dropwise while stirring, and reflux in a hot water bath for 2 hours. After the reaction was completed, part of the solvent was removed by rotary evaporation, and an khaki solid was precipitated, filtered with suction, washed with a small amount of frozen water, and dried to obtain 2.0 g of khaki powder with a yield of 95.2%.
[0030] 2) Add 0.912g R6GH to a 100mL two-necked flask, and then add 35mL dichloromethane to completely dissolve R6GH, then cool with a cold water bath. Dissolve 0.1mL (slightly excess) of oxalyl chloride in 10mL of dichloromethane, and add it dropwise to the above-mentioned 100mL two-necked flask under vigorous stirring (Note: R6GH and glass instruments should be dried before use). After the addition, the stirred mixture was placed in a cold water bath for ...
specific Embodiment 2
[0032] Solution preparation:
[0033] 1) Preparation of rhodamine 6G hydrazide oxamide standard solution
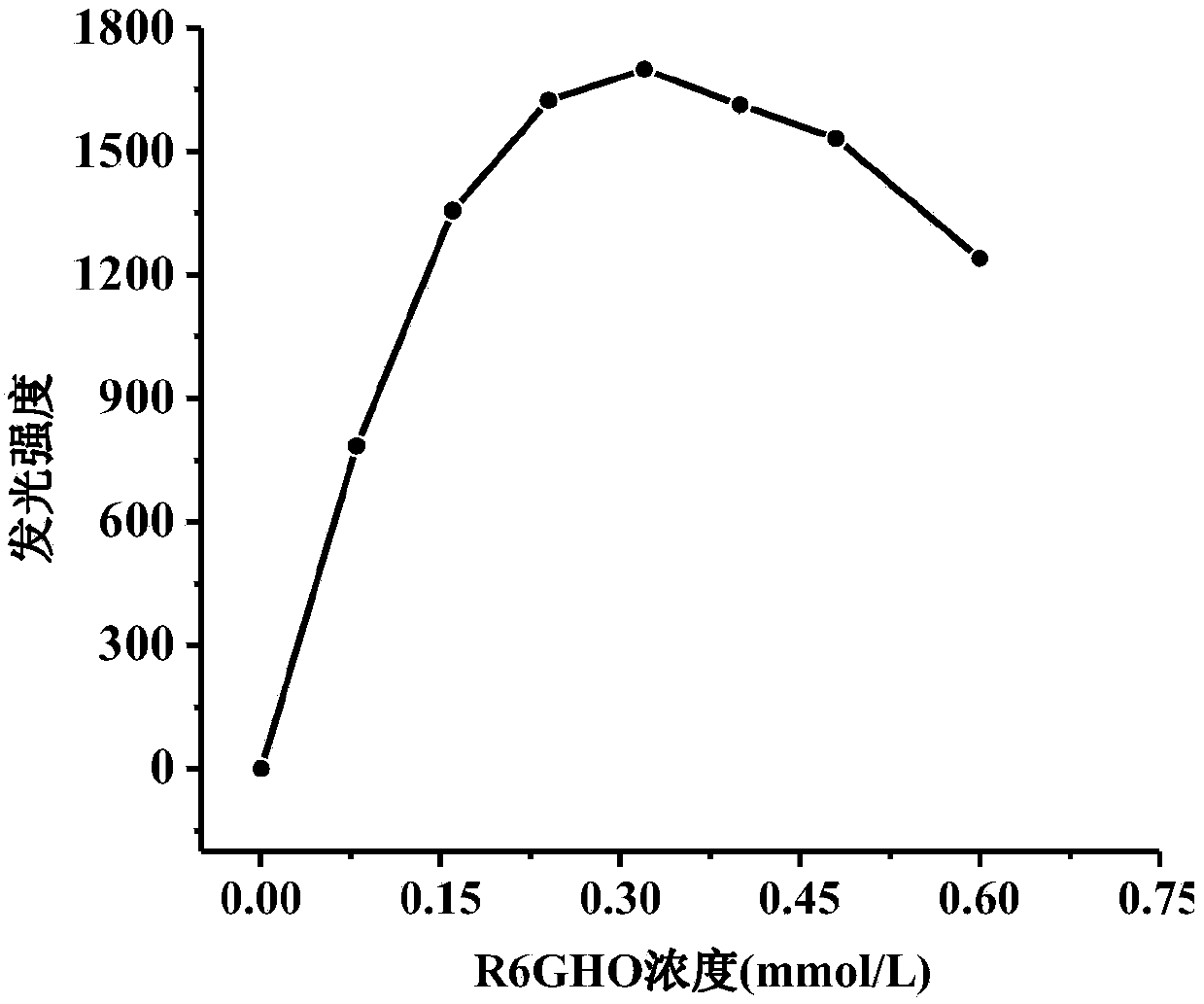
[0034]Accurately weigh 0.2276g of rhodamine 6G hydrazide oxamide (R6GHO) with an analytical balance into a 50ml beaker, add 2ml of 2mol / L HCl solution, stir with a thin glass rod to completely dissolve R6GHO, then add an appropriate amount of deionized water, and then transfer to In a 250ml volumetric flask, wash the small beaker and thin glass rod, and transfer the washing solution into the above-mentioned volumetric flask together, and finally make it to volume. The concentration of the resulting R6GHO standard solution is 1.00 × 10 -3 mol / L.
[0035] 2) Preparation of sodium nitrite (NO 2- )standard solution
[0036] Use a pipette to accurately pipette 10.00ml of 10.0mmol / L sodium nitrite solution into a 100ml volumetric flask, set the volume to the mark, shake well and put it in a dark place to obtain 1.0mmol / L sodium nitrite (NO 2- ) stock solution.
[0037] R6...
Embodiment 3
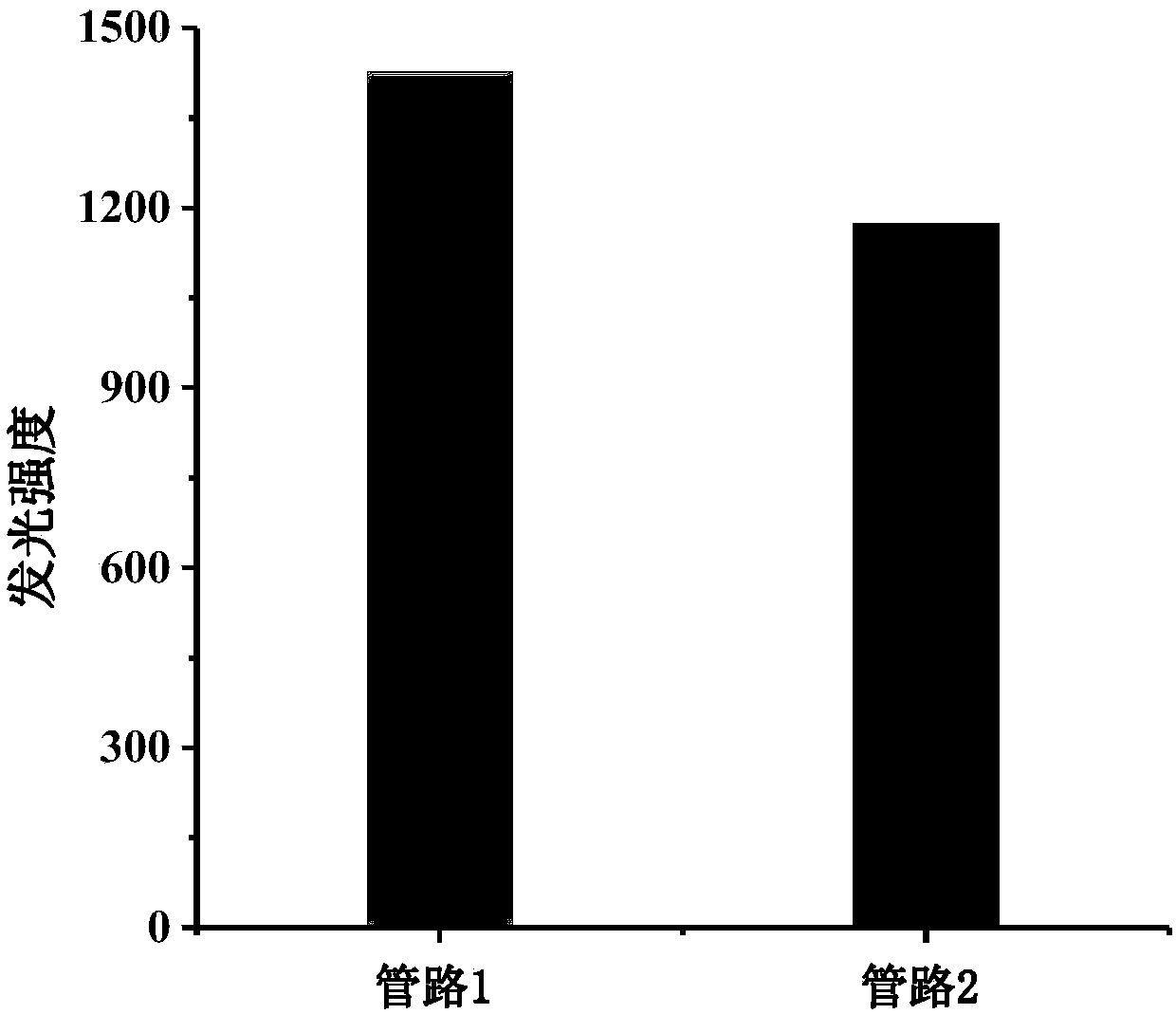
[0045] Determination of NO in saliva by the chemiluminescence system of the present invention 2- content:
[0046] Sample Processing: Saliva samples were obtained from four volunteers who were asked to rinse their mouths before collecting the samples. The collected samples were first centrifuged at a speed of 12000r / min for 10min to remove the protein in the saliva, and then 1ml of the supernatant was taken in a 100ml volumetric flask for later use.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com