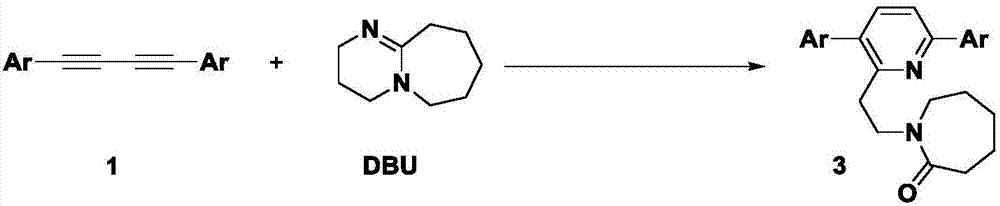
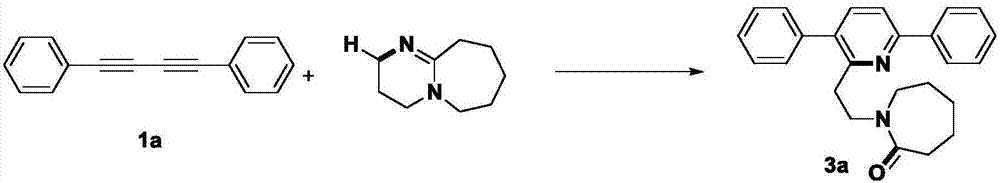
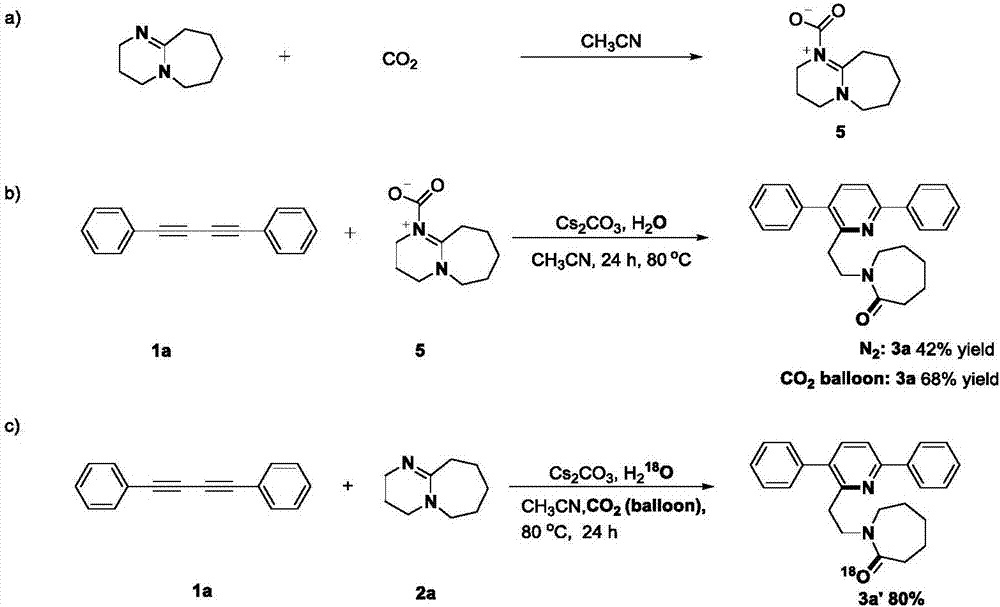
Method for synthesizing alpha-ethyl azacycloheptane-2-one substituted pyridine compounds
A technology of heterocycloheptane and compounds, applied in the field of organic synthesis, to achieve the effect of avoiding the use of transition metal catalysts and toxic ligands, less experimental steps, and low technical difficulty
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-8
[0032] Under a carbon dioxide atmosphere, 0.2 mmol 1,3-butanediyne, 0.2 mmol cesium carbonate, 0.6 mmol DBU, a small amount of water and 2 mL acetonitrile were successively added to a Schlenk reaction tube, and the reaction was heated and stirred at a constant temperature in IKA for 24 h. After the reaction was completed, it was cooled to room temperature, 20 mL of ethyl acetate was transferred to the reaction solution, the sample was prepared by rotary evaporation under reduced pressure, and the target product 3a-3h was obtained by column chromatography separation.
[0033]
[0034]
[0035] In this Example 1-8, a variety of 1,4-diphenylbutadiynes substituted by electron withdrawing and electron donating groups at the para and meta positions of the benzene ring and 1,3-butadiynes substituted by heterocycles and DBU's response. According to the above experiments, it can be found that the reaction has a wide range of substrate adaptability to the alkyl, methoxy, fluoro, c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com