Preparation method and application of polyethylene glycol-deoxycholic acid and derivatives of polyethylene glycol-deoxycholic acid
A technology of polyethylene glycol and deoxycholic acid, which is applied in the direction of drug combination and antineoplastic drugs, can solve the problems of chemotherapy failure and other problems, and achieve good biocompatibility, good anti-multidrug resistance, good The effect of applying the foreground
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032]
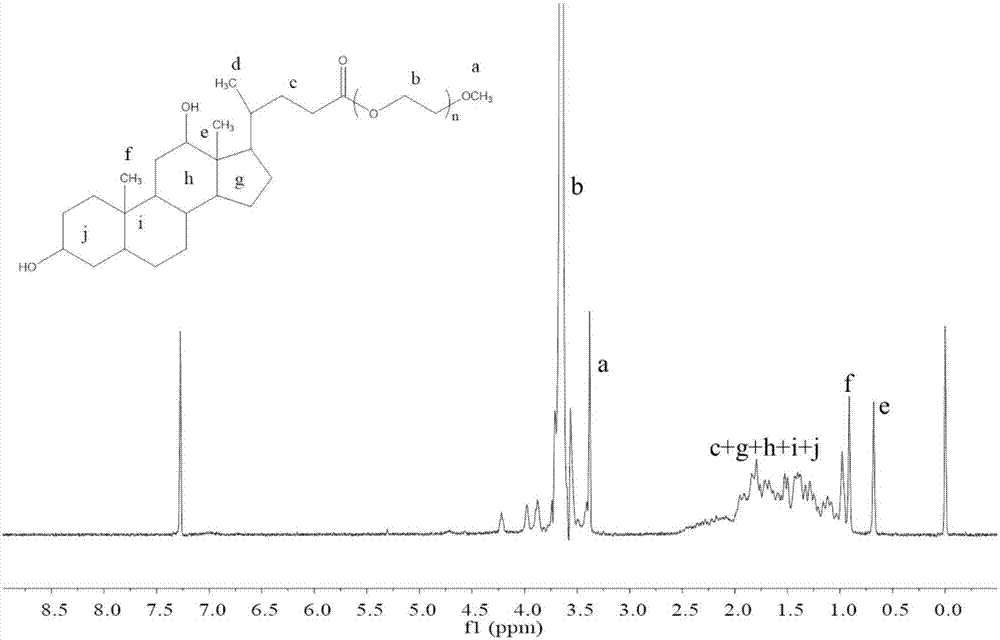
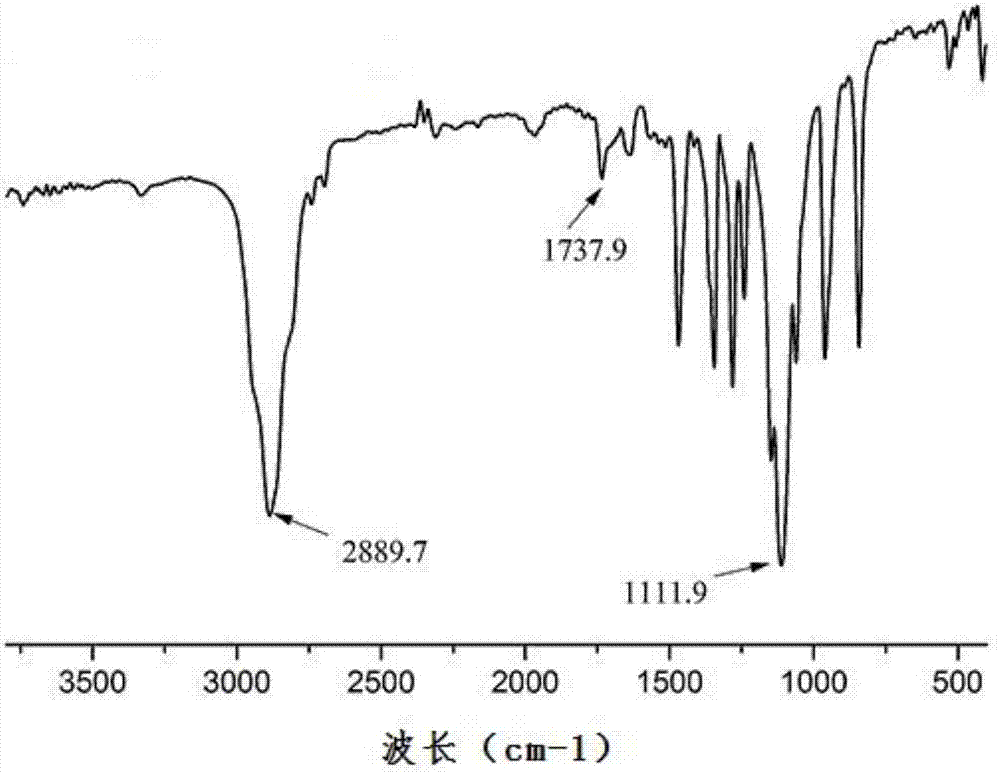
[0033] Weigh 1mmol of deoxycholic acid and 1.5mmol of DMAP in a 100mL eggplant-shaped bottle, then add 40mL of anhydrous dichloromethane (CH 2 C l2 ) stirred at room temperature for 30min, rotating speed 600r / min, then weighed 1.1mmol polyethylene glycol and dissolved it in 5mL of dichloromethane, then added it dropwise in the reaction flask, then added 1.6mmol of DCC (dicyclohexyl Carbodiimide), stirred at room temperature for 24h, after the reaction was completed, suction filtered, extracted once with 0.5mol / L hydrochloric acid, then extracted twice with saturated brine, and the organic layer was washed with anhydrous magnesium sulfate (MgSO 4 ) drying, suction filtration, rotary evaporation to concentrate the solvent, anhydrous ether for precipitation, standing overnight, suction filtration, and vacuum drying at 37°C to obtain polyethylene glycol-deoxycholic acid (PEG-DCA).
[0034] The infrared and 1H NMR images of the resulting polyethylene glycol-deoxycholic...
Embodiment 2
[0037]
[0038] 1. Synthesis of 3,3'-dithiodipropionic anhydride
[0039] Weigh 5g of 3,3'-dithiodipropionic acid and place it in a vacuum drying oven to dry at 30°C, and then take another 50mL of acetyl chloride and re-distill it at 75°C. Add the freshly distilled acetyl chloride to the dried 3,3'-dithiodipropionic acid, and reflux at 70°C for 2 hours. After the reflux is completed, the excess acetyl chloride is evaporated under reduced pressure, and the residue is precipitated by adding excess ice anhydrous ether. Place in the lower layer of the refrigerator to freeze overnight at -20°C, filter with suction the next day, and dry the precipitate in a vacuum oven at 45°C for 10 hours. A light yellow to grayish yellow flaky solid was obtained with a yield of about 60%.
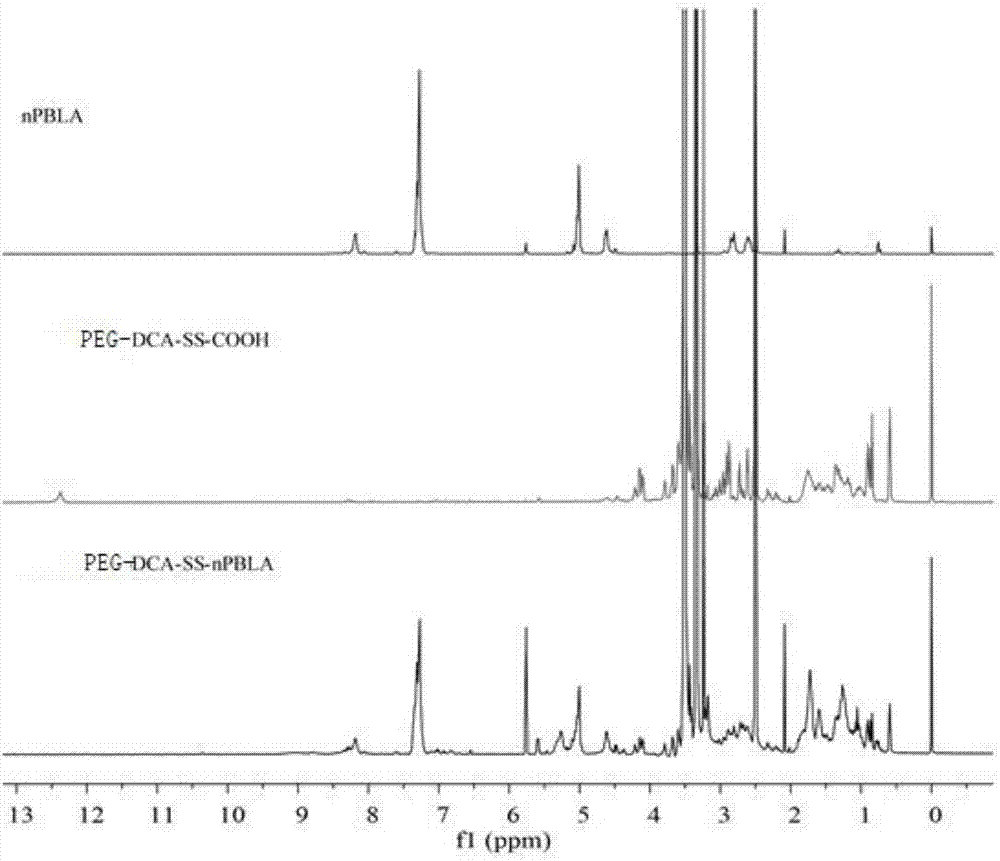
[0040] 2. Weigh 1.07g (5.6mmol) of the above-mentioned synthesized 3,3'-dithiodipropionic anhydride, place in a 100mL round-bottomed three-necked flask, add 40mL of anhydrous DMF, and 2 After protecting an...
Embodiment 3
[0047]
[0048] 1. Synthesis of PEG-DCA-DTPA
[0049] Weigh 0.5mmol of dithiodipropionic anhydride and place it in a three-neck flask filled with 25mL of dichloromethane, stir under nitrogen protection until all dithiodipropionic anhydride is dissolved, weigh 0.1mmol PEG-DCA, 0.5mmol DMAP In 8 mL of dichloromethane, the mixture was added dropwise into a three-necked flask, and then 4 drops of triethylamine were added, and reacted at 35° C. for 24 h. The reaction solution was precipitated with ether, and the precipitate was dissolved in saturated sodium bicarbonate to generate a large number of bubbles, then extracted twice with dichloromethane, the organic phase was discarded, and the pH was adjusted to about 2 with 0.1mol / L hydrochloric acid, and then Extracted twice with dichloromethane, combined the organic phases, dried over anhydrous magnesium sulfate, concentrated the solvent, precipitated with diethyl ether, filtered with suction, and dried under vacuum at 37°C to ob...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com