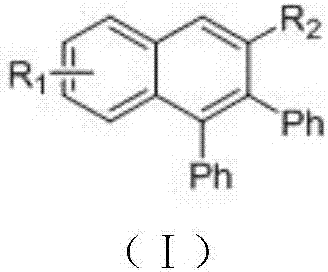
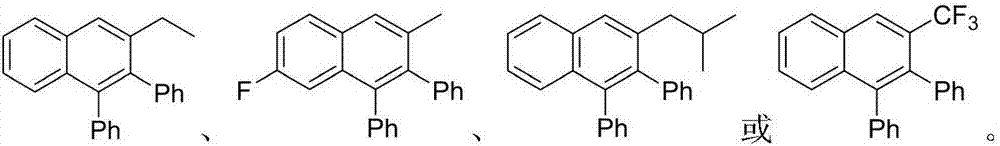
Method for preparing polyaromatic substituted naphthalene derivative by catalyzing cyclization reaction of aromatic ketone and diphenyl acetylene by ruthenium and application
A technology of tolanyl and naphthalene derivatives, which is applied in the field of preparation of polyaromatic substituted naphthalene derivatives, can solve problems such as increasing production costs, and achieve the effect of simple and easy synthesis methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021]
[0022] To a 25mL sealed tube with a magnet, add toluene (18mg, 0.1mmol), the corresponding aromatic ketone (0.2mmol), catalyst [RuCl 2 (p-cymene)] 2 (9mg, 15% mol), 0.5mL toluene, then add dry sodium carbonate (21mg, 0.2mmol) and potassium acetate (19mg, 0.2mmol), replace nitrogen three times, react at 100°C for 24 hours, then pass column chromatography Separation (eluent: petroleum ether) to obtain the target compound. Characterization is as follows.
[0023] 4,5-Diphenyl-6-(thiophene-2-methylene)benzo[b]thiophene: Yield: 40%. 1 H NMR (CDCl 3 ,400MHz)δ7.81(s,1H),7.33(d,J=5.2Hz,1H),7.10-7.18(m,9H),7.00-7.02(m,3H),6.87(dd,J 1 = 3.6Hz; J 2 =5.2Hz,1H),6.59-6.60(m,1H),4.07(s,2H). 13 C NMR (CDCl 3 ,100MHz)δ144.1,139.7,139.4,135.7,130.8,130.4,127.5,126.7,126.5,126.4,125.9,124.2,123.8,122.1,34.5. 25 h 18 S 2 (M + ):382.0850,found:382.0847.
Embodiment 2
[0025]
[0026] To a 25mL sealed tube with a magnet, add toluene (18mg, 0.1mmol), the corresponding aromatic ketone (0.2mmol), catalyst [RuCl 2 (p-cymene)] 2 (9mg, 15% mol), 0.5mL toluene, then add dry sodium carbonate (21mg, 0.2mmol) and potassium acetate (19mg, 0.2mmol), replace nitrogen three times, react at 100°C for 24 hours, then pass column chromatography Separation (eluent: petroleum ether) to obtain the target compound. Characterization is as follows.
[0027] 3-Methyl-1,2,7-triphenylnaphthalene: Yield: 65%. Melting point: 163-165°C. 1 H NMR (CDCl 3 ,400MHz)δ7.91(d,J=8.4Hz,1H),7.79(s,1H),7.69-7.73(m,2H),7.52(d,J=7.2Hz,2H),7.37(t,J =8.0Hz, 2H), 7.29(d, J=7.2Hz, 1H), 7.10-7.23(m, 8H), 7.02-7.05(m, 2H), 2.26(s, 3H). 13 CNMR (CDCl 3 ,100MHz)δ141.5,140.6,140.4,139.2,139.0,137.9,134.7,132.1,131.5,131.1,130.1,128.8,127.7,127.6,127.5,127.4,127.2,127.1,126.4,120.5HR (EI-TOF) calcd for C 29 h 22 (M + ):370.1722,found:370.1723.
Embodiment 3
[0029]
[0030] To a 25mL sealed tube with a magnet, add toluene (18mg, 0.1mmol), the corresponding aromatic ketone (0.2mmol), catalyst [RuCl 2 (p-cymene)] 2 (9mg, 15% mol), 0.5mL toluene, then add dry sodium carbonate (21mg, 0.2mmol) and potassium acetate (19mg, 0.2mmol), replace nitrogen three times, react at 100°C for 24 hours, then pass column chromatography Separation (eluent: petroleum ether) to obtain the target compound. Characterization is as follows.
[0031] 3-Ethyl-1,2-diphenylnaphthalene: Yield: 25%. Melting point: 124-125°C. 1 H NMR (CDCl 3,400MHz)δ7.79-7.81(m,1H),7.72(s,1H),7.37-7.40(m,2H),7.22-7.26(m,1H),7.01-7.14(m,8H),6.96- 6.98(m,2H),2.51(dd,J 1 =7.6Hz;J 2 =14.8Hz, 2H), 1.08(t, J=7.6Hz, 3H). 13 C NMR (CDCl 3 ,100MHz)δ140.4,140.3,139.6,139.5,138.8,133.0,131.2,131.0,130.4,127.4,127.4,126.8,126.3,126.1,125.8,125.7,125.3,27.4,15.1. 24 h 20 (M + ):308.1565,found:308.1567.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com