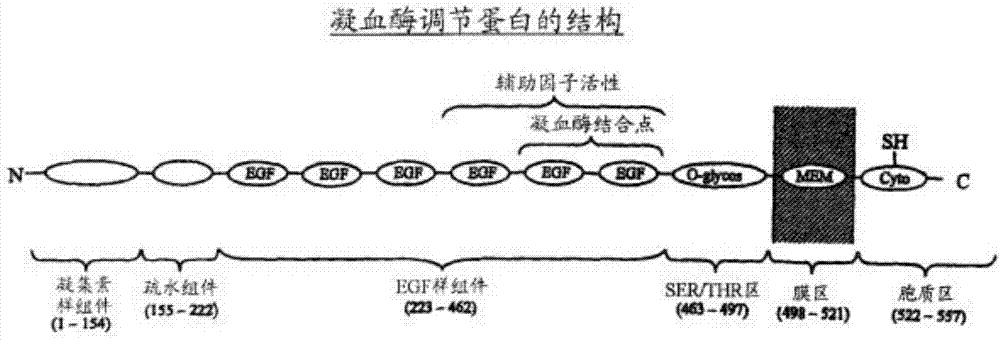
Preparation method of human derived thrombomodulin capable of being industrially produced
A technology for regulating protein and urinary protein, which is applied in the field of preparation of human-derived hemagglutination regulatory protein, can solve the problems of low yield and unsuitability for large-scale industrial production, and achieve high biological activity, industrialization basis, and high yield. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
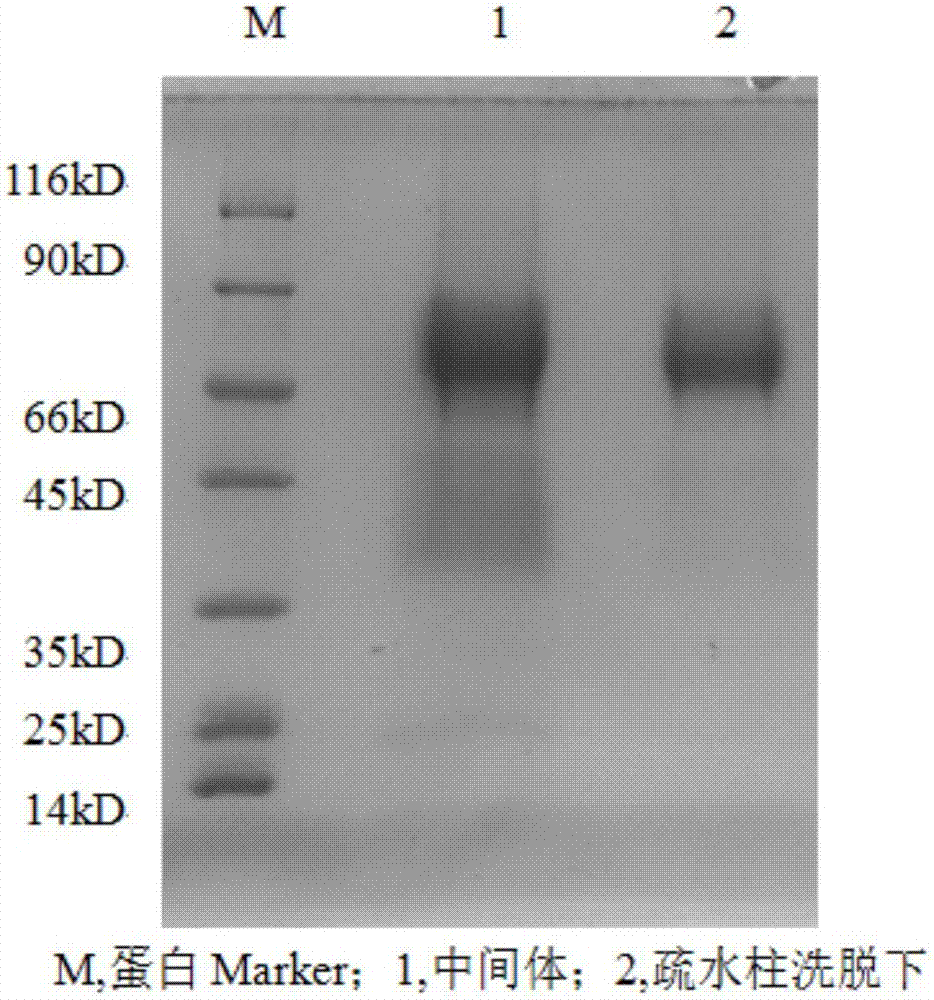
Examples
Embodiment 1
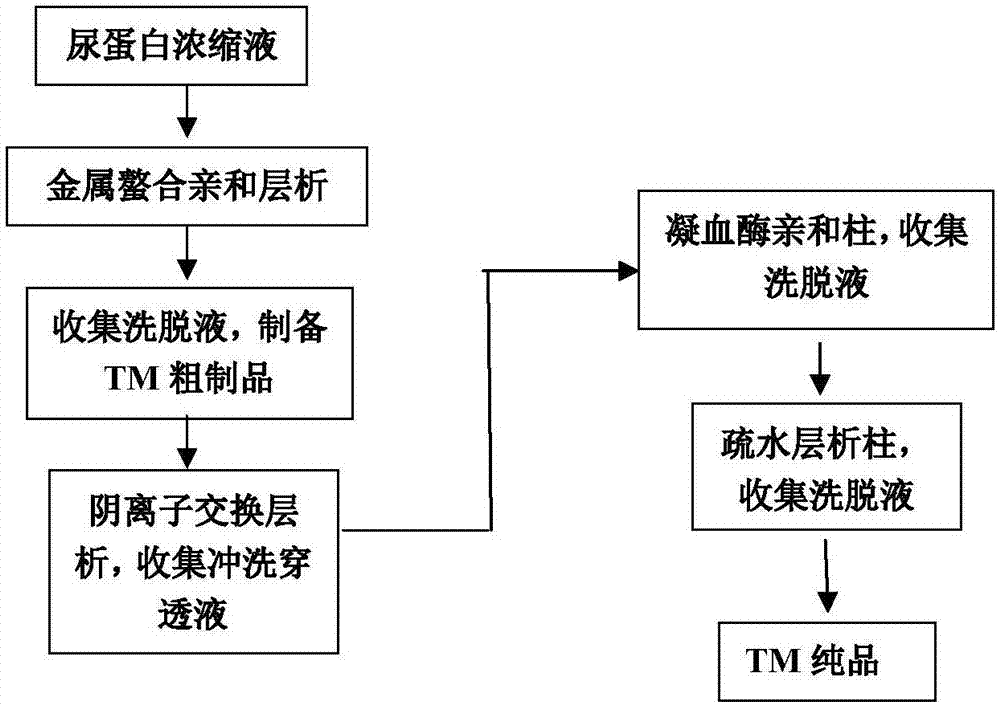
[0029] Collect 100 kilograms of resins that absorb urine protein, wash with water, pack column; Carry out elution with the NaCl aqueous solution of 0.5mol / L, collect elution peak, record the content of hemagglutinin wherein is 11mg.
[0030] a. Metal chelate affinity chromatography
[0031] Use an ultrafiltration membrane with a molecular weight cutoff of 30K for ultrafiltration concentration, adjust the pH of the ultrafiltration concentrate to 5.0, and the conductivity to 0.5mS / cm, and the metal ion that has been balanced on the surface is Cu 2+ The metal chelating affinity chromatography column (Chelating sepharoseFF); then balance liquid (equilibrium liquid formula: 0.2mol / L acetate buffer solution, containing 0.05mol / LNH 4 Ac, pH is 5.0) to wash the metal chelate affinity chromatography column, collect the sample loading and wash the breakthrough, wash with the washing solution (0.2mol / L acetate buffer, containing 0.1mol / LNH 4 Ac, pH is 5.0) washing, with eluent (0.2mol / L...
Embodiment 2
[0042] Collect 100 kilograms of resins that absorb urine protein, rinse with water, pack into columns; carry out elution with the NaCl aqueous solution of 1.5mol / L, collect elution peak, measure the content of hemagglutinin wherein is 10mg.
[0043] a. Metal chelate affinity chromatography
[0044] Use an ultrafiltration membrane with a molecular weight cut-off of 30K for ultrafiltration concentration, adjust the pH of the ultrafiltration concentrate to 8.0, and the conductivity to 10mS / cm, and the balanced metal ion on it is Zn 2+ The metal chelate affinity chromatography column (Chelating sepharoseFF), and then balance liquid (equilibrium liquid formula: 0.02mol / L phosphate buffer, containing 0.5mol / LNH 4 Ac, the pH is 8.0) to wash the metal chelate affinity chromatography column, collect the sample loading and washing breakthrough, and use the washing solution (0.02mol / L phosphate buffer, containing 0.5mol / LNH 4 Ac, pH is 8.0) washing, with eluent (0.02mol / L phosphate aque...
Embodiment 3
[0054] Collect 5 tons of resin that absorbs urinary protein, rinse with water, pack column; Carry out elution with the NaCl aqueous solution of 0.8mol / L, collect elution peak, record the hemagglutinin content wherein is 500mg.
[0055] a. Metal chelate affinity chromatography
[0056] Use an ultrafiltration membrane with a molecular weight cutoff of 30K for ultrafiltration concentration, adjust the pH of the ultrafiltration concentrate to 6.0, and the conductivity to 5.0mS / cm, and the metal ion that has been balanced on the surface is Cu 2+ The metal chelating affinity chromatography column (Chelating sepharoseFF); then use the equilibrium solution (equilibrium solution formula: 0.1mol / L phosphate buffer, containing 0.1mol / LNH 4 Ac, pH is 6.0) to wash the metal chelate affinity chromatography column, collect the sample loading and wash the breakthrough, wash with the flushing solution (0.1mol / L phosphate buffer, containing 0.2mol / L NH 4 Ac, pH 6.0) wash, with eluent (0.1mol / L...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com