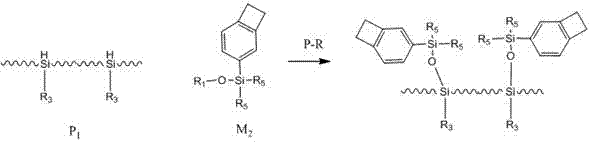
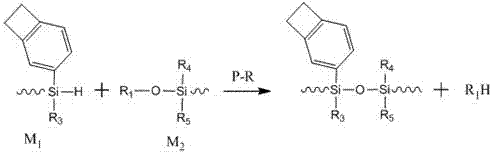
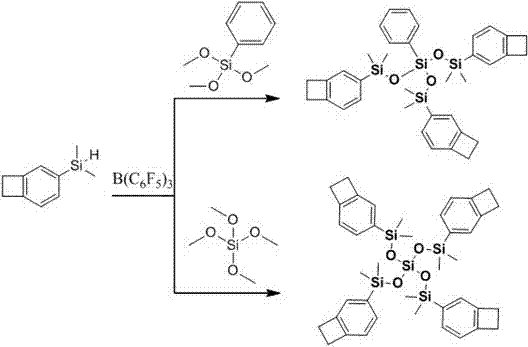
Benzocyclobutene functional organic silicone resin and preparation method thereof
A benzocyclobutene functional, benzocyclobutene technology, applied in the field of silicone resin and its preparation, can solve problems such as low glass transition temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] Preparation of tribenzocyclobutene-functionalized silicones by reaction of benzocyclobuten-4-yldimethylsilane and phenyltrimethoxysilane
[0066] .
[0067] Add benzocyclobuten-4-yldimethylsilane (0.70g), tris(pentafluorophenyl)borane (2.32mg), and 4ml of toluene into a 50ml three-necked flask equipped with a stirring bar and nitrogen protection, Ice bath. Slowly drop 0.285 g of phenyltrimethoxysilane into the three-necked flask. After the addition is complete, a large number of bubbles will be generated in the reaction system. After the bubbles disappeared, the temperature was raised to 60°C for 4 hours of reaction. After the reaction, cool to room temperature, add neutral alumina, filter, and spin the filtrate to dryness with a rotary evaporator. Using petroleum ether / ethyl acetate as the mobile phase, it was purified by silica gel column chromatography to obtain 0.61 g of a colorless transparent liquid (yield 66%).
Embodiment 2
[0069] Preparation of tribenzocyclobutene-functionalized silicones by reaction of benzocyclobuten-4-yldimethylsilane and phenyltrimethoxysilane
[0070] .
[0071] Add benzocyclobuten-4-yldimethoxysilane (0.70g), tris(pentafluorophenyl)borane (2.32mg), and 4ml of toluene into a 50ml three-necked flask equipped with a stirring bar and protected by nitrogen ,Ice bath. Slowly drop 0.22 g of tetraethyl orthosilicate into the three-necked flask. After the addition is complete, a large number of bubbles will be generated in the reaction system. After the bubbles disappeared, the temperature was raised to 60°C for 4 hours of reaction. After the reaction, cool to room temperature, add neutral alumina, filter, and spin the filtrate to dryness with a rotary evaporator. Using petroleum ether / ethyl acetate as the mobile phase, it was purified by silica gel column chromatography to obtain 0.52 g of a colorless transparent liquid (yield 65%).
Embodiment 3
[0073] Add 1,4-bis(dimethylsilyl)benzene (1.944g), tris(pentafluorophenyl)borane (8mg), and 10ml of toluene into a 50ml three-necked flask equipped with a stirring bar and protected by nitrogen. Add methyldimethoxy(benzocyclobuten-4-yl)silane (2.203g) and 10ml of toluene into a constant pressure dropping funnel and mix well. At room temperature, the mixture was dripped into a three-necked bottle, and the dripping was completed in one hour. After the addition was complete, the temperature was raised to 60°C for 4 hours of reaction. After the reaction, cool to room temperature, add neutral alumina, filter, and spin the filtrate to dryness with a rotary evaporator. 1 H NMR (500 MHz, CDCl3, δ): 7.514 (s, 4H), 7.403-7.351 (t, 1H), 7.215-7.185 (d, 1H), 6.988-6.973 (d, 1H), 6.146-5.978 (m , 2H), 5.887-5.784 (m, 1H), 3.203-3.129 (t, 4H), 0.399-0.241 (m, 12H)
[0074] .
[0075] Molecular weight is analyzed by GPC test MP=6811, polydispersity=1.015937.
PUM
| Property | Measurement | Unit |
|---|---|---|
| dispersity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com