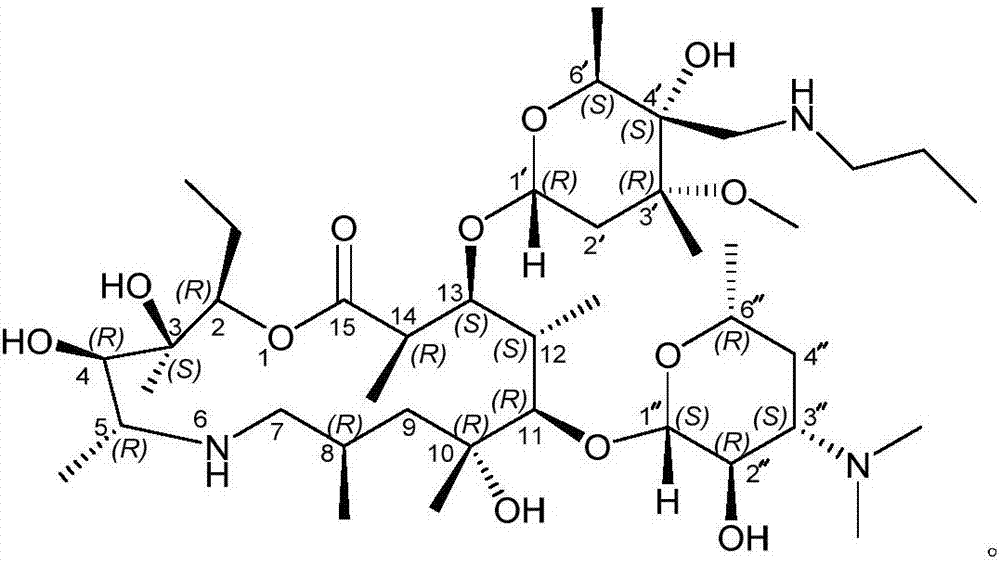
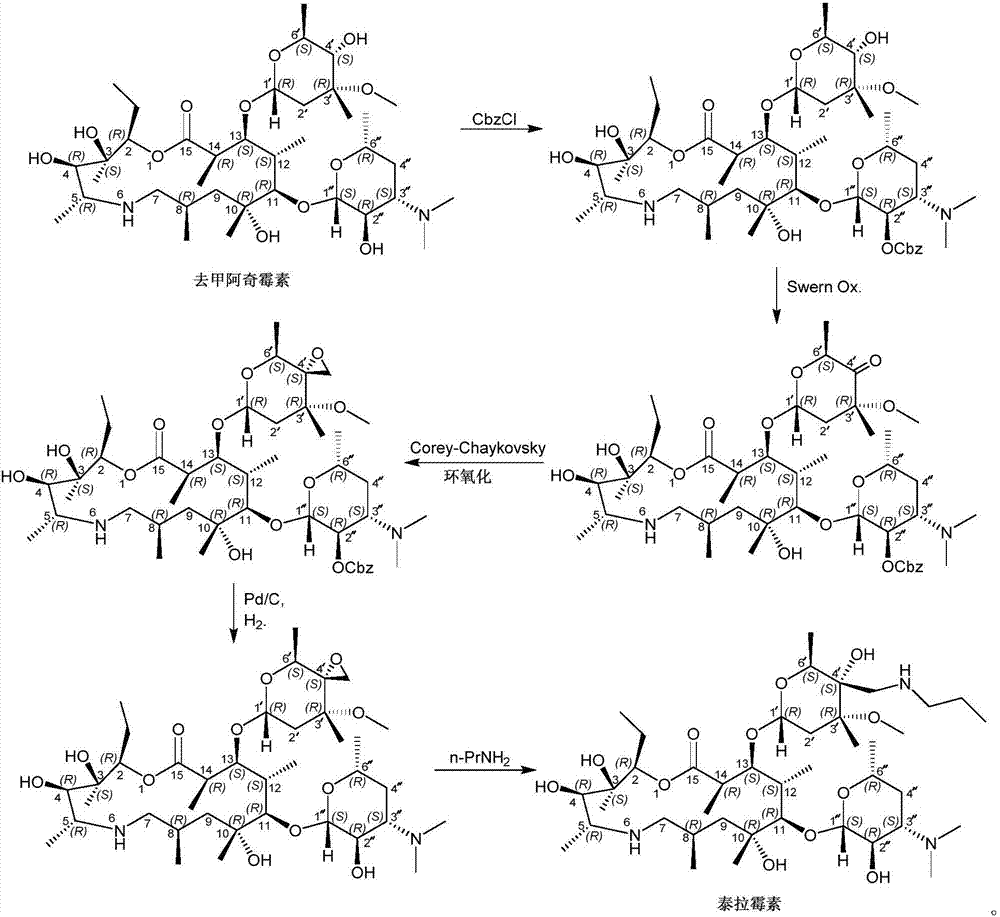
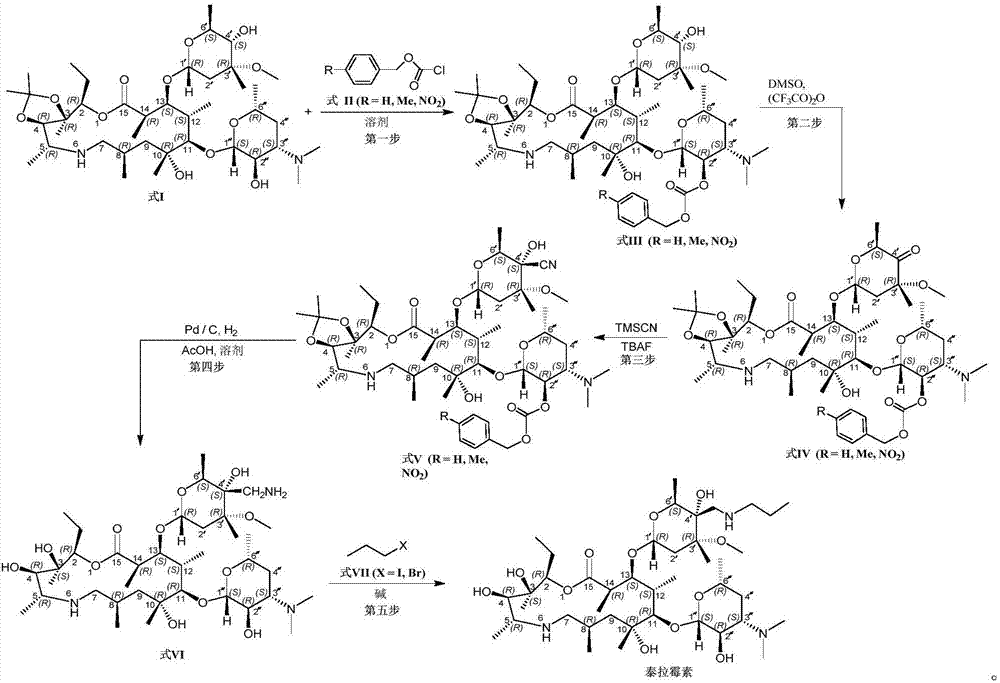
Method for preparing tulathromycin
A telamycin and synthesis method technology, applied in the preparation of sugar derivatives, chemical instruments and methods, sugar derivatives, etc., can solve the problems of many impurities in the reaction, poor selectivity, difficult to purify, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0034] 1, preparation formula III (R=Me) compound
[0035] Add compound formula I (500g, 645.16mmol) and CH 2 Cl 2 (3.5L), the system was fully stirred to maintain the system temperature at about 20 degrees. Slowly add p-methylbenzyl chloroformate (formula II, R=Me, 180 g, 975 mmol) dropwise to the reaction system through the dropping funnel, and maintain the temperature of the system at about 20 degrees during the dropping process. After the dropwise addition, the system was incubated for 3 hours. The system was slowly added with 4 L of saturated aqueous sodium bicarbonate solution to quench the reaction, and after the addition was complete, the system was stirred at room temperature for 2 hours. The system was left to stand for 2 hours, separated, the organic phase was washed with 1 L of water, and then 250 g of anhydrous sodium sulfate was added to the organic phase, stirred and dried. After filtration, the organic phase was concentrated under reduced pressure until the...
Embodiment 7
[0049] Compound IV (R=NO) prepared in embodiment 7 2 , 25g, 26.3mmol) was added to a 500mL reaction flask, and CH was added to the system 2 Cl 2 (180mL), after stirring and dissolving, the temperature of the reaction system was cooled to about 0°C in an ice-salt bath, and then trimethylsilylcyanide (3.3g, 33.3mmol) was slowly added through a syringe. After the addition, the system was naturally warmed up to room temperature and reacted overnight, then slowly added TBAF (1M in THF, 35mL) dropwise to the system, and the reaction was incubated for 3 hours after the dropwise addition was completed. Slowly add H to the reaction system 2 O (100 mL) quenched the reaction, the system was left to stand and separated, the organic phase was separated, the organic phase was washed twice with saturated brine (2×80 mL), and the organic phase was dried over anhydrous sodium sulfate. Filter, concentrate the organic phase under reduced pressure until there is no obvious fraction, add ethyl ...
Embodiment 8
[0051] Compound V (R=NO) prepared in embodiment 8 2 , 16g, 16.3mmol) was added to a 500mL small autoclave, then isopropanol (120mL) and HOAc (45mL) were added, and after stirring to dissolve, Pd / C (2.5g, 10%) was added under nitrogen protection. The system was replaced with nitrogen three times, and the hydrogenation reaction was carried out at 35° C. and 5 atm for 24 hours. After the reaction was complete, the reaction solution was taken out, filtered twice through a Buchner funnel, the filter cake was washed with isopropanol (20 mL), the filtrate was combined, the filtrate was concentrated under high vacuum and reduced pressure until no obvious fraction was present, and ethyl acetate (10 mL) was added to the residue. , stirred to dissolve, slowly added heptane (50mL) dropwise, stirred overnight, a large amount of white solids precipitated out of the system, filtered, and the filter cake was washed with a little cooled n-heptane and dried to obtain the compound of formula VI ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com