P-aminobenzene sulfonic acid metal complex antibacterial agent as well as preparation method and application thereof
A technology of p-aminobenzenesulfonic acid and metal complexes, which is applied in the direction of antifungal agents, antibacterial drugs, zinc organic compounds, etc., and can solve the severe toxicity of sulfanilamide compounds, the difficulty of synthesizing sulfanilamide compounds, and the insufficient antibacterial activity of sulfanilamide compounds Strong and other problems, to achieve the effect of good inhibitory activity, good inhibitory activity, and outstanding antibacterial effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Synthesize the p-aminobenzenesulfonate titanium complex used in this embodiment as follows
[0052]
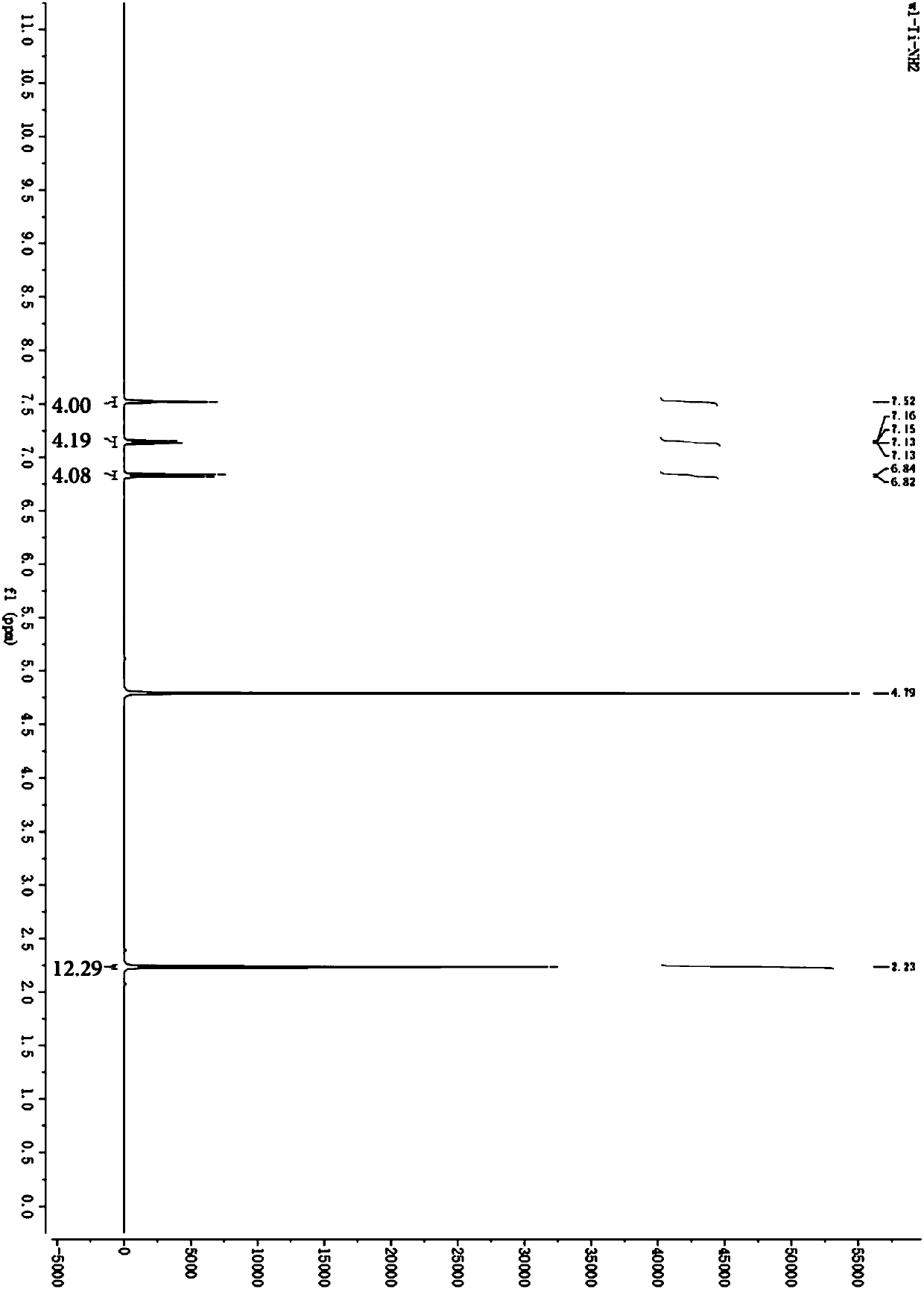
[0053] Dissolve 1.54g of 4-amino-3-methylbenzenesulfonic acid (Ⅲ) in 10mL of ethanol, and slowly add 5mL of an ethanol solution with a concentration of 0.14g / mL tetrabutyl titanate (II) dropwise at room temperature. Stir at 70°C for 3-5 hours, then cool to room temperature, remove the solvent by rotary evaporation, and the residual solid is the target product (IV). 1 H NMR (400MHz,D 2 O) δ 7.52 (s, 4H), 7.14 (dd, J = 8.2, 1.6 Hz, 4H), 6.83 (d, J = 8.2 Hz, 4H), 2.23 (s, 12H). figure 1 For the product (Ⅳ) 1 H NMR spectrum.
Embodiment 2
[0055]
[0056] Synthesize the p-aminobenzenesulfonate zinc complex used in this embodiment as follows
[0057] Dissolve 1.87g of 4-amino-3-methylbenzenesulfonic acid (Ⅲ) in 15mL of ethanol, add 400mg of NaOH solid, stir at room temperature for half an hour, add 940mg of anhydrous zinc nitrate (Ⅴ), stir at 50-70°C for 3 ~5 hours, after cooling, the solvent was distilled off under reduced pressure, and the remaining solid was the target product (Ⅵ). 1 H NMR (400MHz,D 2 O) δ 7.56 (s, 2H), 7.16 (d, J = 8.1 Hz, 2H), 6.88 (d, J = 8.1 Hz, 2H), 2.28 (s, 6H).
Embodiment 3
[0059]
[0060] Synthesize the p-aminobenzenesulfonate silver complex used in this embodiment as follows
[0061] Dissolve 2.03g of 4-amino-3-methoxybenzenesulfonic acid (Ⅷ) in 15mL of ethanol, add 123mg of silver oxide solid, stir at 60-70°C for 3-5 hours, filter after cooling, and distill off the filtrate under reduced pressure solvent, and the remaining solid is the target product (IX). 1 H NMR (400MHz,D 2 O) δ 7.54 (s, 1H), 7.08 (dd, J=8.0, 1.4Hz, 1H), 6.85 (d, J=8.0Hz, 1H), 3.75 (s, 3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com