A kind of biomimetic small peptide ligand-based affinity-enriched monolithic material and its preparation and application
A technology of monolithic materials and peptide ligands, applied in the field of affinity enrichment monolithic materials, can solve the problems of insufficient specific selectivity, toxicity and biocompatibility, and achieve the effects of low price, low non-specific adsorption, and simple preparation methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
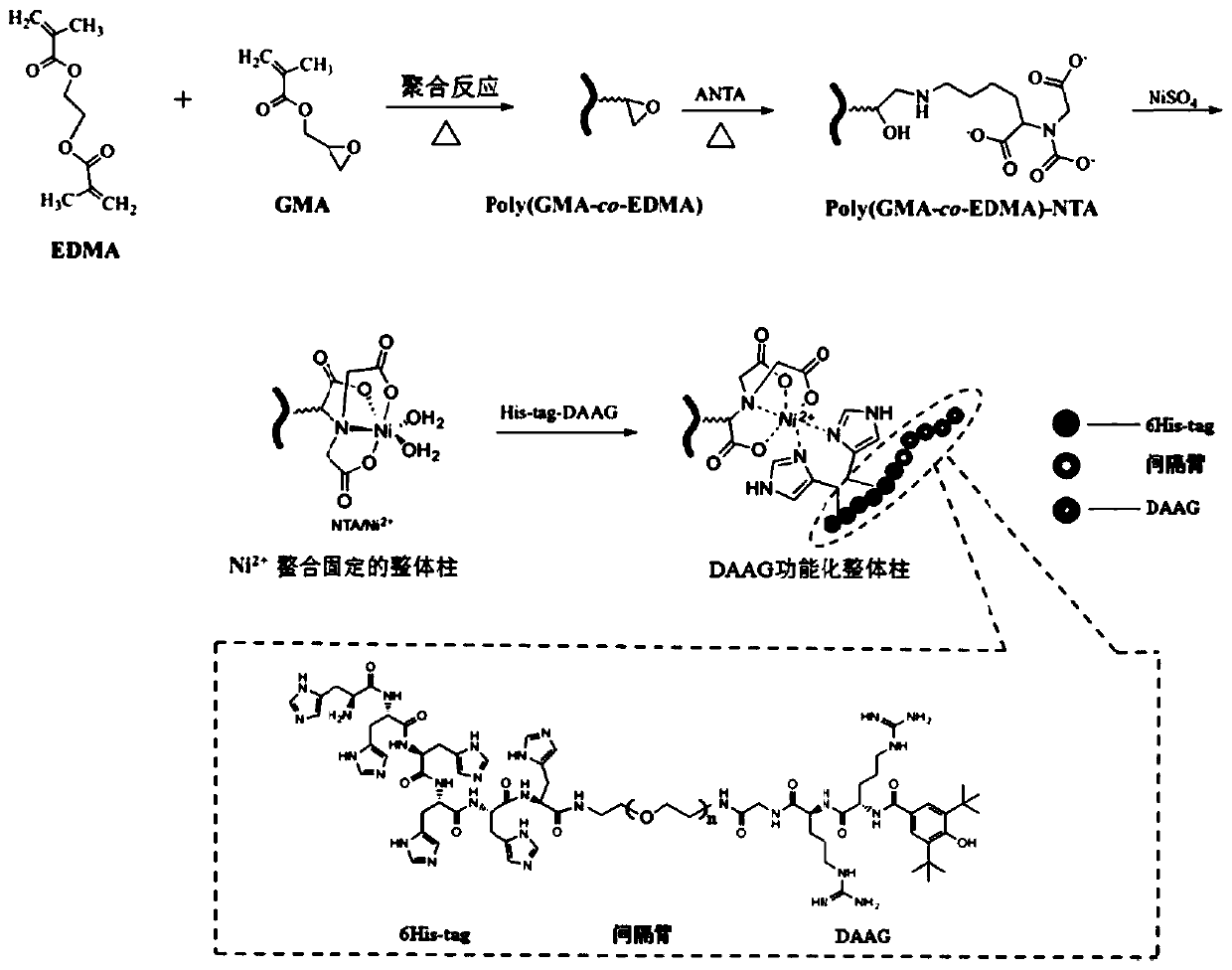
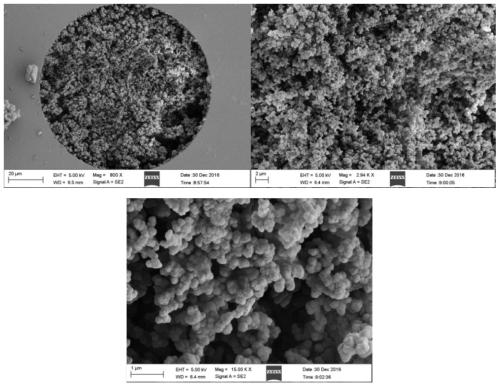
[0047] (1) Preparation of the matrix monolithic column: the monomer GMA (glycidyl methacrylate), the cross-linking agent EDMA (ethylene dimethacrylate), the porogen (water, 1,4-butanediol and n-propylene Alcohol mixed system) and initiator AIBN (azobisisobutyronitrile), according to the ratio of the literature [Analytical chemistry, 2015,87 (8): 4552-4559.] prepared into a polymerization reaction mixed solution, after ultrasonic dissolution, degassing Pour into the quartz capillary pretreated by γ-MAPs, then seal both ends of the quartz capillary, and put it in a 65°C water bath to react for 12 hours; after the reaction, connect the quartz capillary to a high-pressure pump to wash away unreacted monomer porogens and oligomers, resulting in poly(GMA-co-EDMA) based monolithic columns.
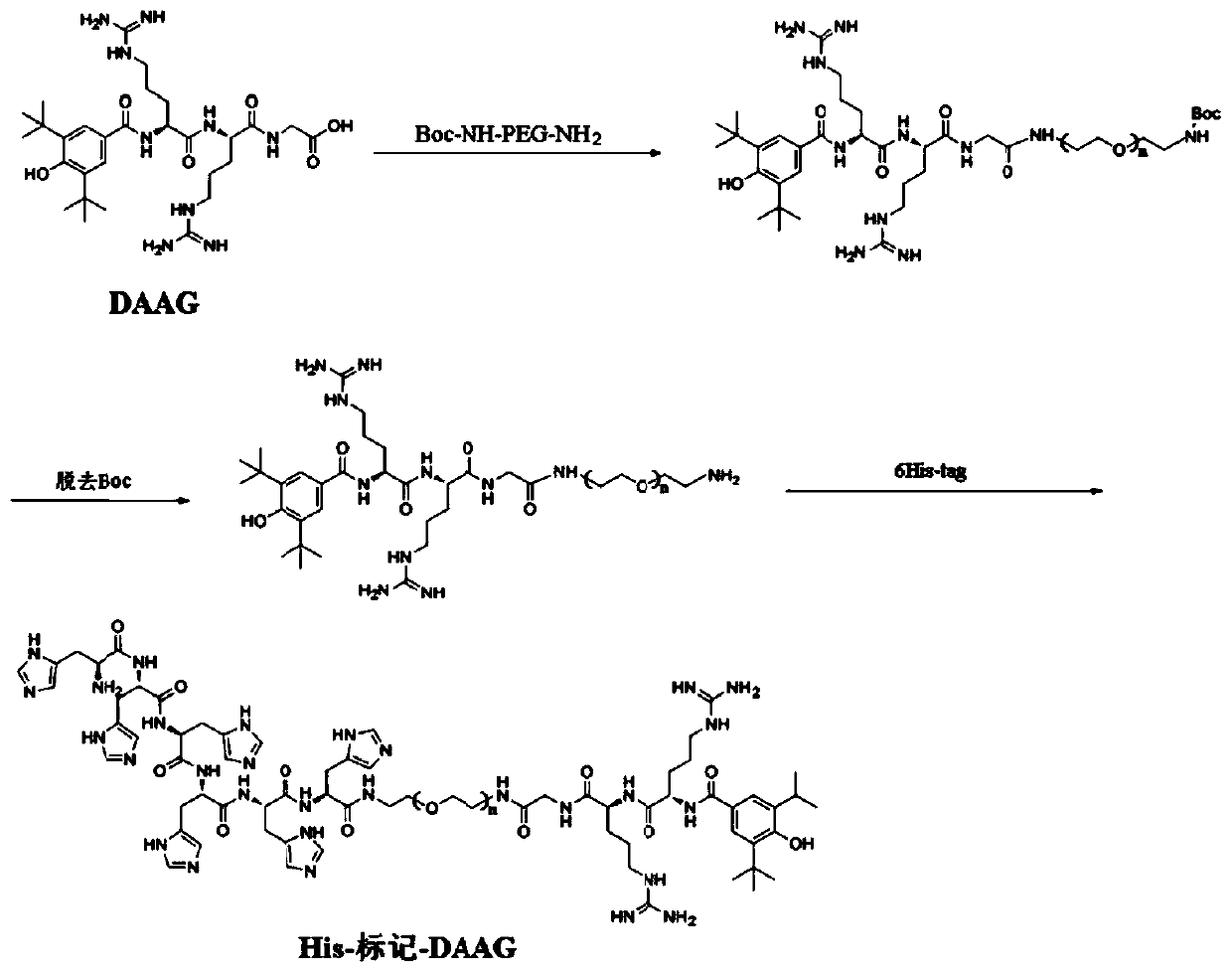
[0048] (2) Preparation of a small peptide ligand containing 6 histidine tags (His-tag-DAAG): The DAAG small peptide was combined with polyethylene glycol diamine (Boc-NH- PEG-NH 2 ) condensatio...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com