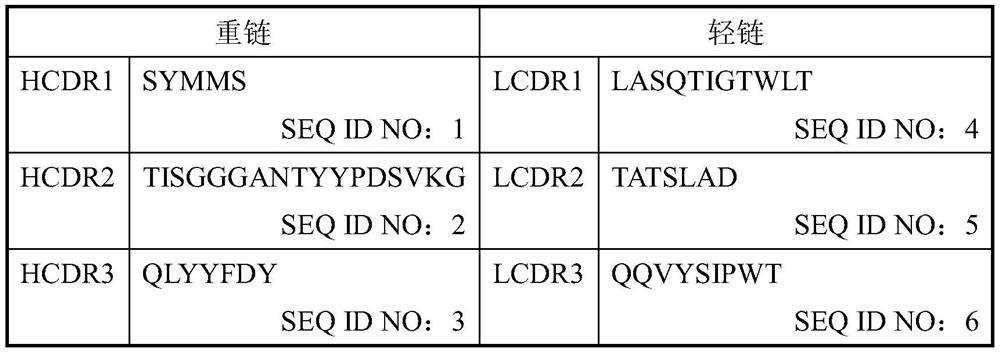
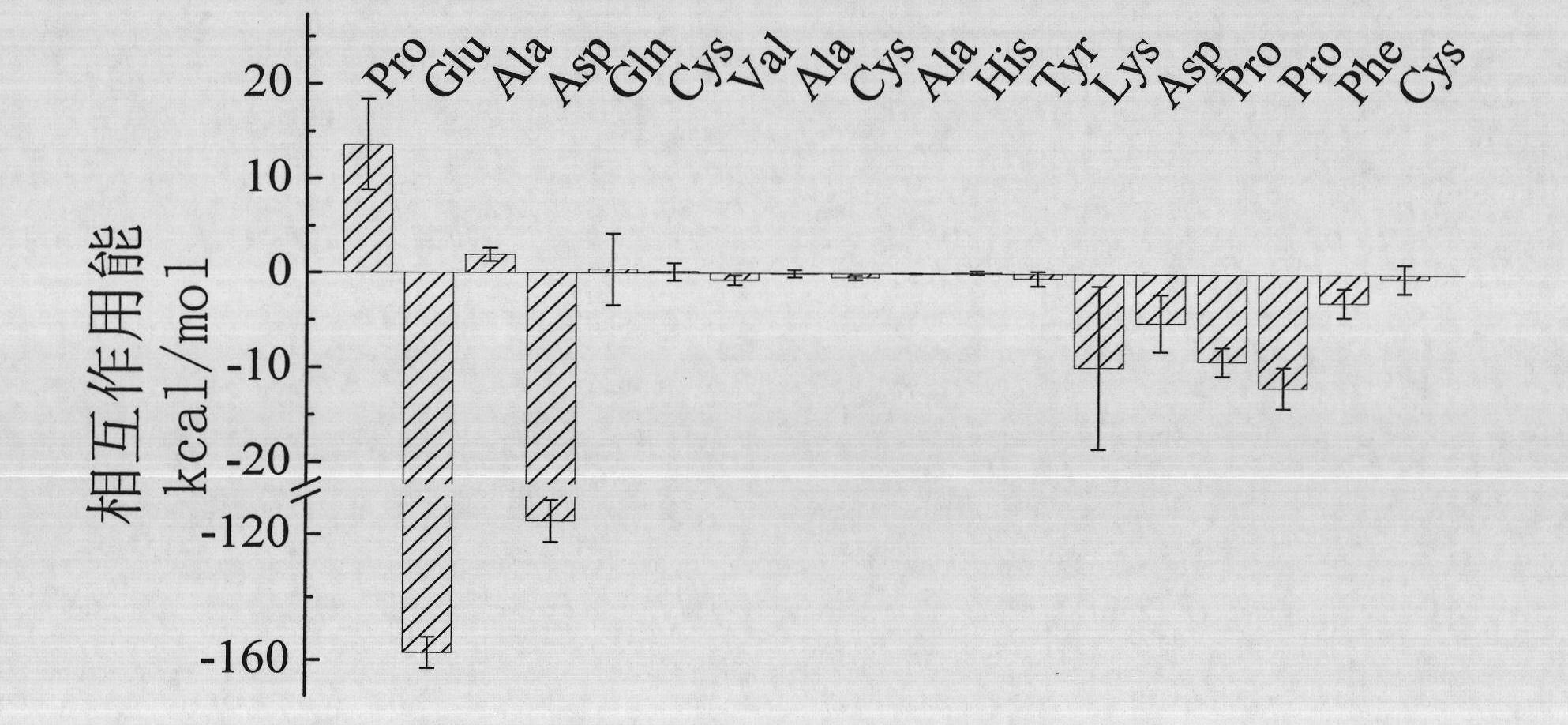
Patents
Literature
32results about How to "Mild elution conditions" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Macropore high-capacity agarose gel media preparing method
InactiveCN1472002AIncrease mass transfer rateLarge adsorption capacityIon-exchange process apparatusComponent separationMicrosphereMicroparticle
A process for preparing high-capacity microreticular agarose gel medium includes such steps as dispersing the granular calcium carbonate as hole-forming agent in the aqueous solution of agarose to obtain suspension, adding it to salad oil, adding emulsifier Span80, emulsifying while stirring, quick cooling for sphericizing, adding epoxy chloropropane, cross linking, reducing with sodium borohydride, and using diluted hydrochloric acid to remove granular calcium carbonate.
Owner:TIANJIN UNIV
Method for purifying lactoferrin from milk serum by using an expanded bed adsorption technology
InactiveCN102898516AReduce investmentReduce lossesTransferrinsPeptide preparation methodsMilk SerumExpanded bed adsorption
The invention provides a method for purifying lactoferrin from milk serum by using an expanded bed adsorption technology. The method comprises the following steps of: (1) adding water into sweet whey powder for dissolving into a whey solution or adjusting the pH of commercially available liquid whey by using NaOH; (2) filling a cation exchanging chromatography medium into an expanded bed, making a phosphate buffer solution pass through a bed layer from bottom to top, and balancing the expanded bed; (3) introducing the solution obtained in the step (1) for adsorbing, eluting by using a buffer solution containing NaCl, and collecting an eluent; and (4) cleaning by using NaOH, and regenerating a medium by using a buffer solution. In the method, expanded bed adsorption is taken as a core separating method, the purity of lactoferrin obtained by one-step separation is up to 88-93 percent, the recovery ratio is 47-86 percent, a process is easy, convenient and efficient, the equipment investment is small, and the method is suitable for industrial production.
Owner:ZHEJIANG UNIV
Performance improved recombination staphylococcus aureus protein A affinity ligand and construction method thereof
ActiveCN103214563AImprove bindingImprove elutionMicroorganism based processesDepsipeptidesHigh concentrationEscherichia coli
The present invention discloses a performance improved recombination staphylococcus aureus protein A affinity ligand and a construction method thereof. The present invention adopts a molecular biology method. A sequence B of a nature protein A is selected for molecular transformation. A C-terminal of the sequence B is added with two cysteines, so that the protein A can pass through double-locus coupled chromatography matrix to stabilize the connection. Six glycines are added to the end of a second Loop of the sequence B to increase the length and reduce the binding force with an antibody, so that elution conditions are mild. On this basis, resistance performance to high concentration base of the protein A is transformed. Asparagines and phenylalanine at 23rd and 30th positions of the sequence B are respectively replaced by threonine and alanine to obtain a sequence Z with higher alkaline resistance properties. Then, isocaudarner is used for connecting sequence Zs of different numbers head-to-tail in series. The efficient expression system of e. coli is used for overexpression. The expressed recombination protein A is coupled to agarose matrix preparation affinity chromatography fillers and is used for purifying antibodies. Results show that the recombination protein A affinity ligand prepared by the present invention is good in elution performance and alkali resistance.
Owner:嘉兴千纯生物科技有限公司
High-capacity dewatering electric charge inducing color chromatogram medium and preparation method
InactiveCN101185882AImprove stabilityEasy to cleanOther chemical processesPeptide preparation methodsEpoxySeparation technology
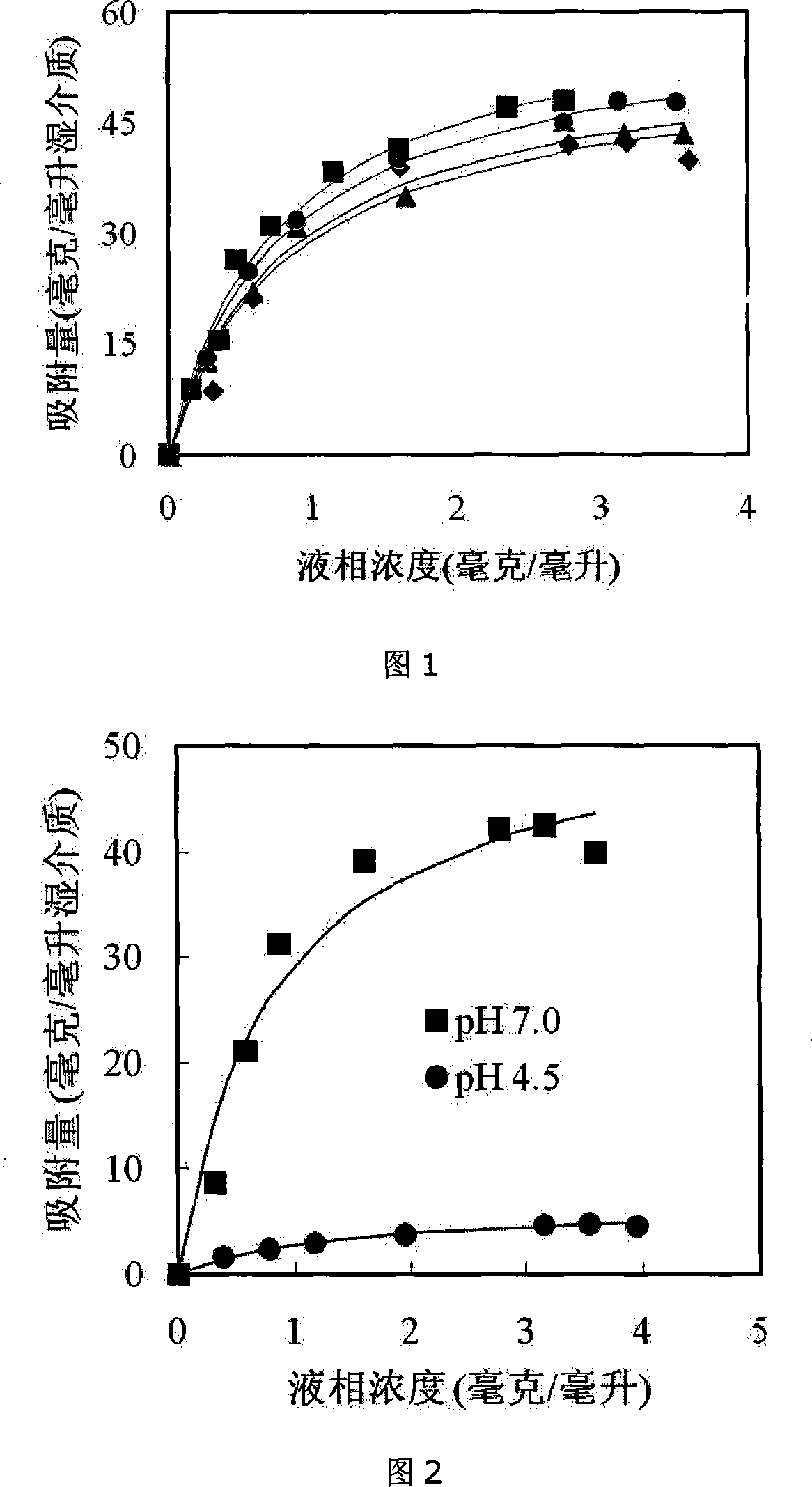
The invention discloses a preparation method of a hydrophobic charge induction chromatography medium with large capacity, and belongs to the protein chromatography separation technology in the field of biochemical engineering. The chromatography medium refers to the end of a space arm that is coupled with imidazol compounds as a chromatography matrix. The preparation method is that: the medium is activated after dimethyl sulfoxide, epichlorohydrin and sodium hydroxide solution are sequentially put into agarose gel medium; the activated medium is coupled with the imidazol compounds matrix in the alkaline dimethyl sulfoxide solution; finally, the epoxy left on the medium surface gets the needed chromatography medium after being reduced. The adsorption capacity of the chromatography medium to the protein achieves a moist medium of 80mg / ml and keeps relatively stable within a broader ionic strength scope from 0.1 mol / L to 1.0 mol / L; at the same time, the protein elutropic model which is finished by depending on the regulation of PH between 4.0 and 6.0 is more moderate, thereby having the wide application prospect in the separation and purification of biomacromolecule such as the protein, etc.
Owner:TIANJIN UNIV
Preparation and application of hexahistidine-tagged protein immunoaffinity purification and enrichment column
InactiveCN103111094AStrong specificityEasy to identifyPreparing sample for investigationSolid sorbent liquid separationPolyclonal antibodiesPolyhistidine-tag
The invention provides an His-tagged recombinant protein immunoaffinity purification and enrichment column capable of efficiently and specifically enriching hexahistidine-tagged protein and a preparation method and application thereof. The provided immunoaffinity purification and enrichment column comprises an activated agarose filler coupled with an immunoaffinity-purified His-specific polyclonal antibody and a plastic column loaded with the immunoaffinity filler, wherein the His-specific polyclonal antibody coupled with the immunoaffinity filler is extracted by an immunoaffinity method. The immunoaffinity purification and enrichment column is prepared by loading the immunoaffinity filler into the special plastic column; and the immunoaffinity purification and enrichment column is high in efficiency and strong in specificity while enriching His-tagged recombinant protein, is mild in diluting conditions, can be repeatedly utilized and is an ideal material for separating, purifying and enriching the His-tagged recombinant protein.
Owner:NANNING LANGUANG BLUE LIGHT BIOTECH +1
Preparation and application of composite TiO*copolymer microspheres
InactiveCN101531734AEasy to operateAppropriate densityOrganic chemistryOther chemical processesBenzoyl peroxideMicrosphere
The invention relates to a method for preparing composite TiO2 copolymer microspheres and application of the composite TiO2 copolymer microspheres in natural product separation and purification. According to the method, an oil phase containing surface-modified TiO2, benzoyl peroxide serving as an initiator, GMA serving as a monomer, DVB serving as a crosslinking agent and the oil phase of a pore forming agent is added into a composite aqueous phase containing gelatin and sodium chloride, and the mixture is heated to cure to form microspheres; and beta-CD is directly immobilized on the microspheres serving as a immobilization matrix in the presence of sodium hydride to form the composite TiO2 copolymer microspheres. The copolymer microspheres can be used as an expanded bed adsorption medium to separate isoflavones from soybean waste molasses and purify the isoflavones. The method has the advantages of simple preparation process and easy control and amplification. The prepared TiO2 copolymer microspheres have the advantages of good medium sphericity, mild elution conditions, high biocompatibility, excellent hydrophilicity and wide application prospect in the natural product separation and purification.
Owner:BEIJING UNIV OF CHEM TECH
Method for preparing microspheric PGDT separating medium with two kinds of pore forms
InactiveCN1351896AImprove hydrophilicityEasy to makeIon-exchange process apparatusOther chemical processesMicrosphereBiological macromolecule
A microspheric PGDT separating medium with two kinds of pore forms is prepared through homogeneously mixing monomer, crosslinking agents, liquid pore-forming agent and initiator; mixing after adding solid pre-forming agent; suspending polymerization to form microballs; ethanol extractino of microballs; acid pickling; and drying. The present invention features the solid pore forming agent calcium carbonat ein the amount of 10-30 vol% of total reactant mixture; the volume ratio between the solid pore-forming agent and the liquid pore-forming agent of 0.5-1; the pore-forming agent amount in 20-60 vol% of the total reactant mixture; the weight ratio between two kinds of crosslinking agents of 0.5-2; the crosslinking agent to monomer weight ratio of 0.3-0.6; and the polymerization temperature under normal pressure controlled at 65-85 deg.C. The said separating medium may be used in separation of protein and other biological macromolecules.
Owner:TIANJIN UNIV
Preparation and application of hemagglutinin peptide mark recombinant protein immunoaffinity purification enriching column
InactiveCN103113455AStrong specificityEasy to identifySolid sorbent liquid separationPeptide preparation methodsHemagglutininPolyclonal antibodies
The invention provides a high-efficiency specificity enriched hemagglutinin peptide HA mark recombinant protein immunoaffinity purification enriching column as well as a preparation method and an application thereof. The immunoaffinity purification enriching column provided by the invention comprises an activated agarose filler coupled with HA specificity polyclonal antibody which is subjected to immunoaffinity purification and a plastic column carrying the immunoaffinity filler. The immunoaffinity purification filler coupled HA specificity polyclonal antibody is extracted by using an immunoaffinity method; and the immunoaffinity purification enrichment column is obtained by carrying the immunoaffinity filler into a specific plastic column. The immunoaffinity purification enrichment column enriched HA mark recombinant protein is high in efficiency, strong in specificity and temperate in elution condition, can be repeatedly utilized and is an ideal material for separating, purifying and enriching the HA mark recombinant protein at present.
Owner:NANNING LANGUANG BLUE LIGHT BIOTECH
Biomimetic monolithic material with affine selectivity similar to that of protein A and preparation method and application thereof
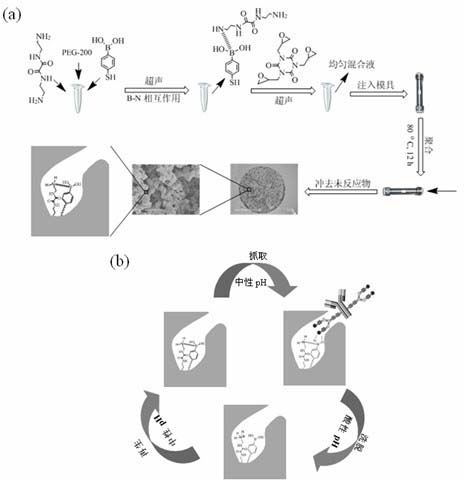
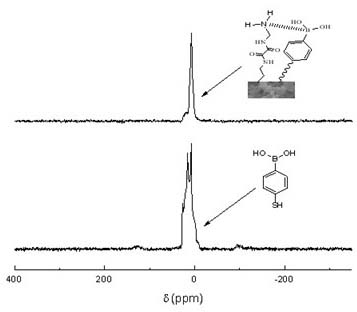
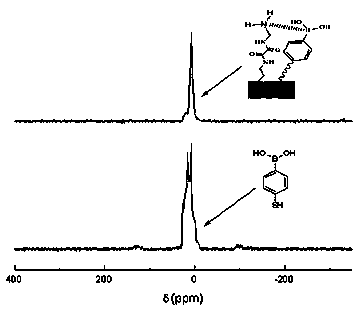
InactiveCN102675602AEasy to makeSafe preparationPeptide preparation methodsImmunoglobulinsEpoxyPhenylboronic acid
The invention discloses a biomimetic monolithic material with the affine selectivity similar to that of a protein A, and a preparation method thereof, wherein the material is capable of purifying, separating and immobilizing antibodies. N, N'-di-(2-aminoethyl) oxamide and 4-mercapto-phenylboronic acid are used as common functional monomers, tri-(2, 3- epoxy group propyl group) isocyanate is used as a monomer, polyethylene glycol is used as a porogenic agent, and reaction is carried out in a mode of in-situ ring-opening polymerization to prepare the biomimetic monolithic material with the affine selectivity similar to that of the protein A. The material can be combined with antibodies specifically, and can be applied to the fields of antibody purification, separation, immobilization and the like. The material has the advantages of high selectivity, low cost, good stability, easiness in elution, reusability and the like, and the immunity affinity and the selectivity of the antibodies are not affected.
Owner:NANJING UNIV
Method for purifying anti-PD-1 antibody
PendingCN112851813AEasy to operateHigh speedImmunoglobulins against cell receptors/antigens/surface-determinantsPeptide preparation methodsAntiendomysial antibodiesVirus inactivation
The invention relates to a method for purifying an anti-PD-1 antibody. Specifically, the purification method comprises the following steps: removing pollutants by using methods such as affinity chromatography, virus inactivation, anionic membrane chromatography, cation exchange chromatography and the like. The process can reduce the production cost and improve the antibody yield.
Owner:SUZHOU SUNCADIA BIOPHARM CO LTD
Affinity peptide specific to herceptin and segment thereof
InactiveCN101955510AGood chemical stabilityNot readily biodegradableImmunoglobulins against cell receptors/antigens/surface-determinantsPeptide preparation methodsSocial benefitsImmunogenicity
The invention discloses an affinity peptide specific to herceptin and a segment thereof, belonging to the technical field of biology. The structure of the peptide is R1-X-R2, wherein R1 is NH2, R2 is COOH, and X is a peptide segment from 3 peptides to 18 peptides and is preferably 4 peptides or 6 peptides. The affinity peptide is coupled to a carrier so as to separate and purify herceptin and the segment thereof. The invention can overcome the defects of high cost, poor stability and insufficient safety of the traditional ligand, and has the advantages of high affinity, high specificity, quick herceptin capture, low production cost, low immunogenicity, high chemical stability and the like. The affinity peptide has excellent application prospects and can produce great social benefits and economical benefits.
Owner:ZHEJIANG UNIV
Method for preparing sialoglycopeptide (SGP) by large-scale separation and purification
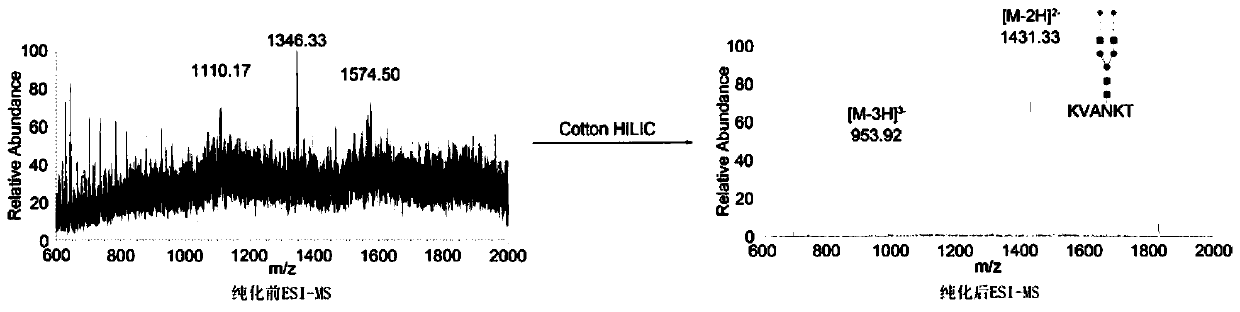
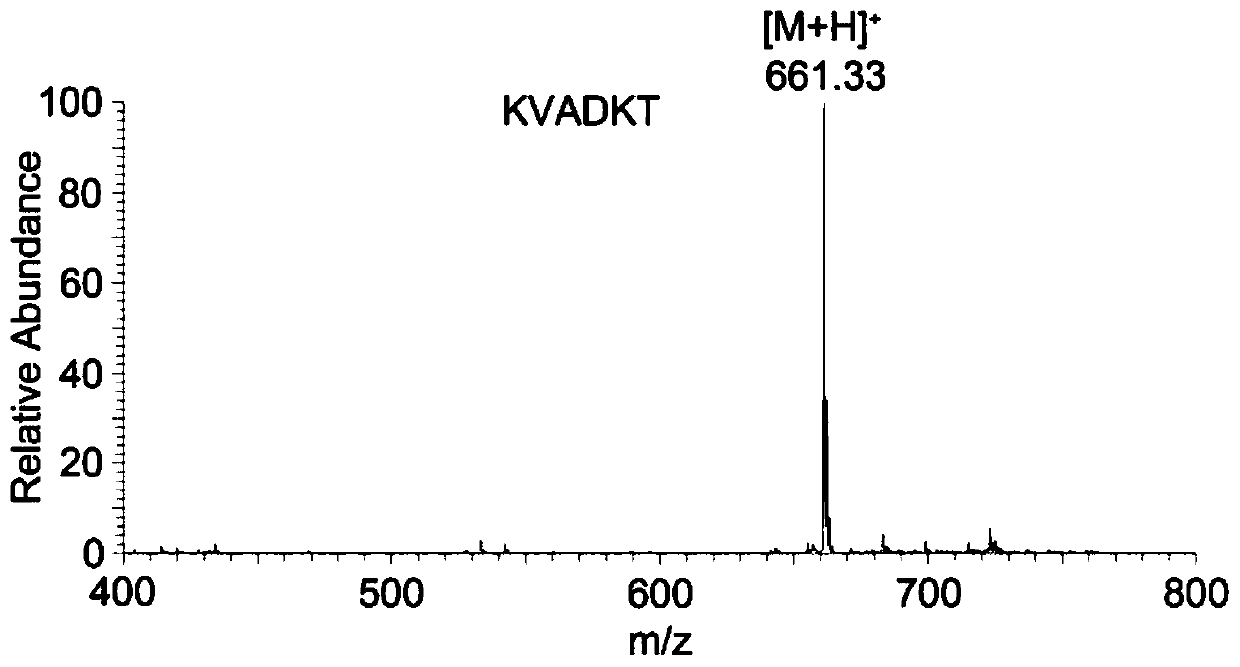
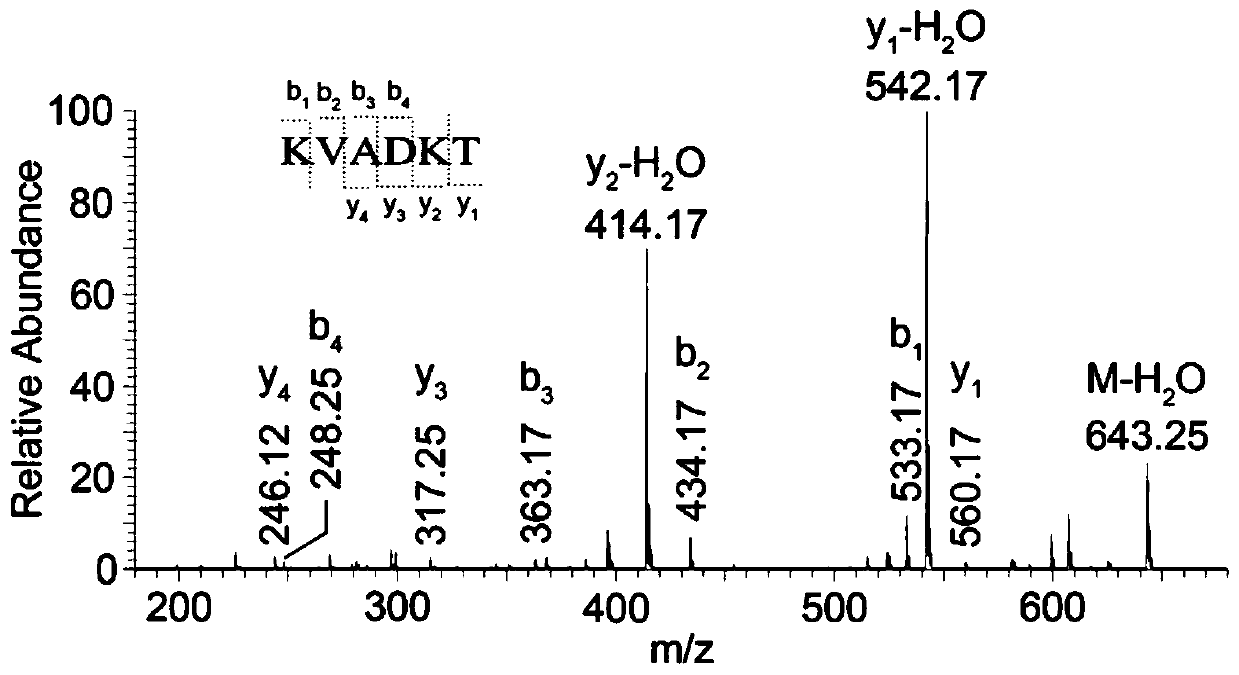
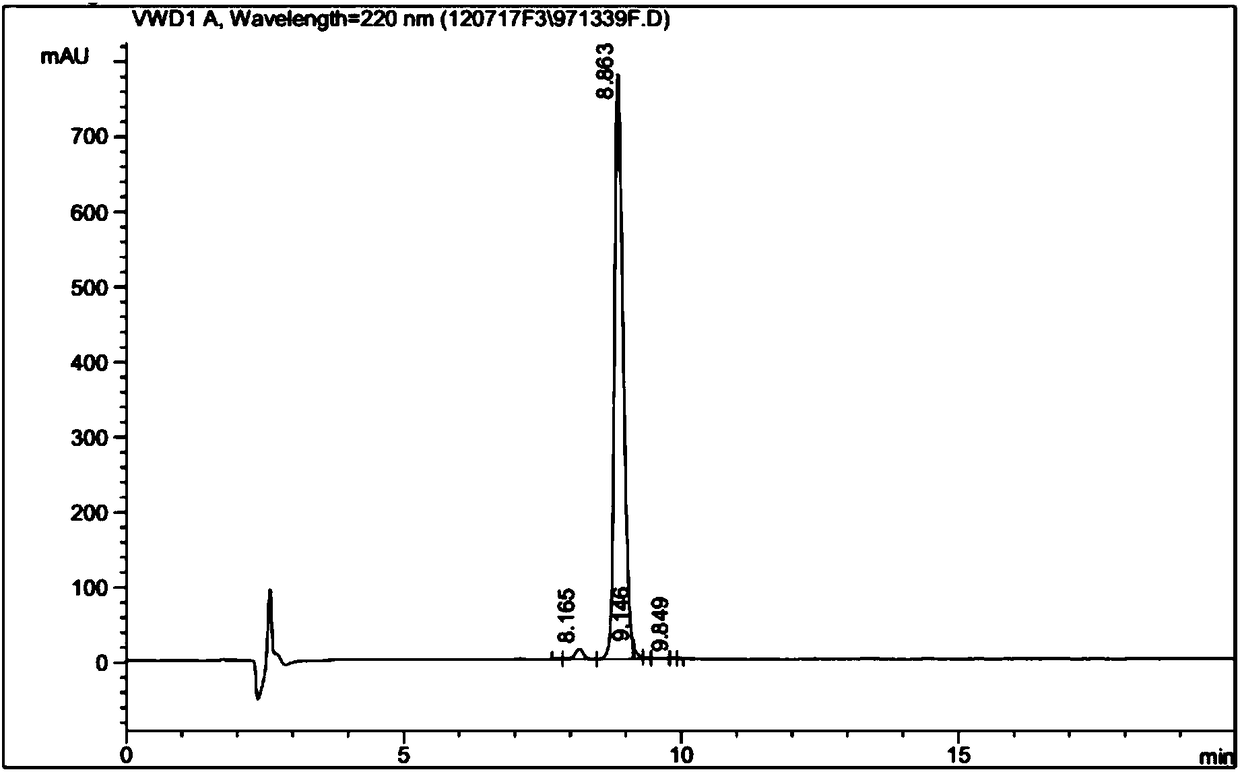
ActiveCN109824762AImprove hydrophilic abilityHigh yieldPeptide preparation methodsAgainst vector-borne diseasesSialoglycopeptidesIon exchange
The invention discloses a method for preparing sialoglycopeptide (SGP) by large-scale separation and purification. The method comprises (1) taking fresh unfertilized egg yolk, treating with phenol, centrifuging to obtain a supernatant and lyophilizing to obtain coarse sialoglycopeptide; (2) filling a cotton hydrophilic chromatographic column with medical degreasing cotton; (3) adding a solution ofthe crude sialoglycopeptide to the cotton hydrophilic chromatographic column; (4) conducting gradient elution on the cotton hydrophilic chromatographic column with acetonitrile to remove salt and impurities and conducting elution and lyophilization on the glycopeptide with water to obtain pure sialoglycopeptide. According to the method, the cumbersome steps of Sephadex G-50 separation, Sephadex G-25 desalination, ion exchange chromatography purification and the like are omitted, the reaction conditions are mild, the experiment cost is reduced, the purification cycle is shortened, the efficiency is improved, and the purity of the sialoglycopeptide is 95% or above according to the purity detection. The method is not only suitable for enrichment of laboratory glycopeptide but also used for industrial production.
Owner:NORTHWEST UNIV(CN)
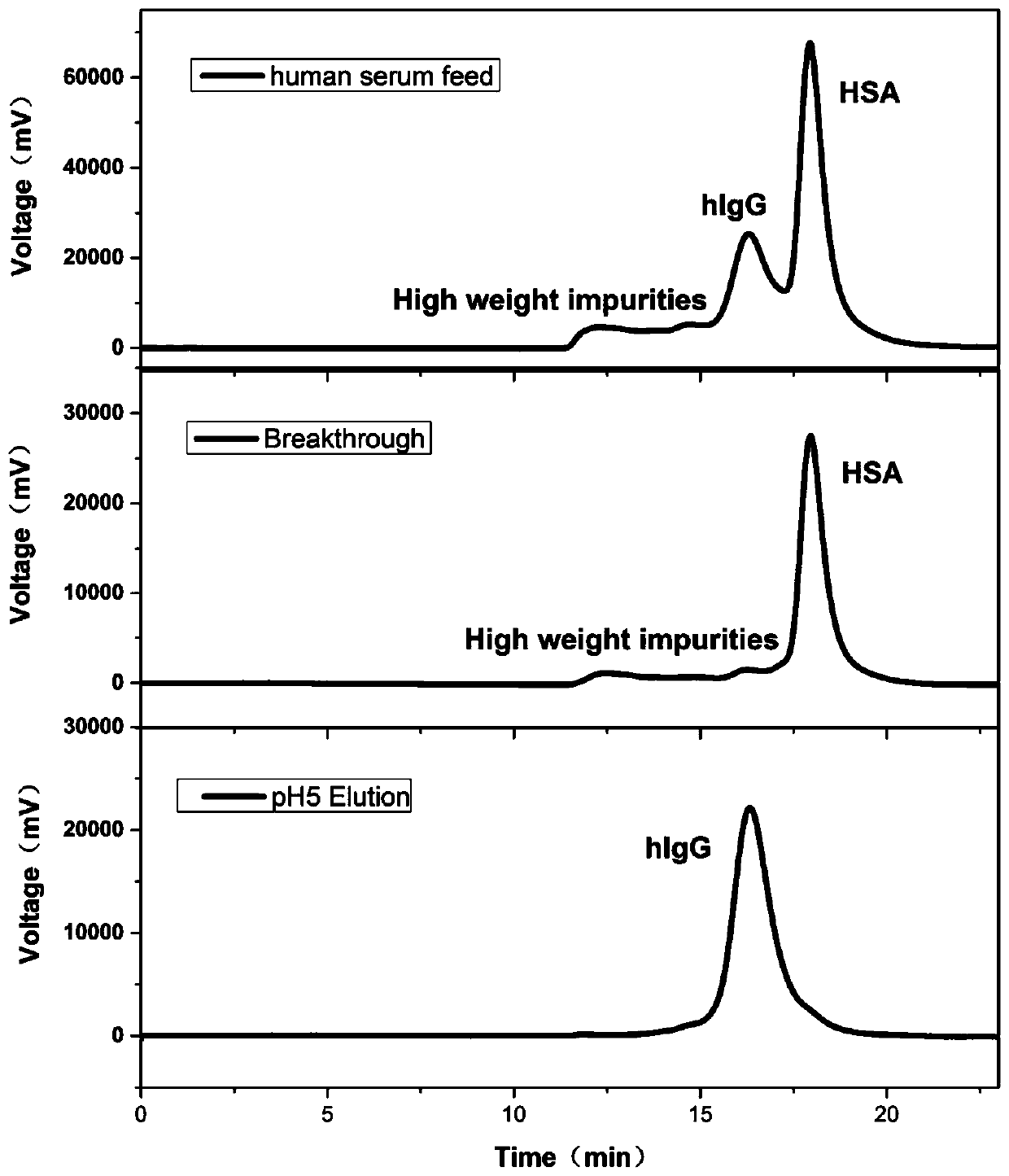
Method for separating immune globulin IgG from human serum
ActiveCN108059673AStrong specificityHigh puritySerum immunoglobulinsPeptide preparation methodsPhenylalanine+TyrosineSerum samples
The invention relates to a method for separating immune globulin IgG from human serum. The method comprises the following steps: 1) diluting the human serum by a buffer solution, adjusting a pH to 6.0to 8.0 and filtering by a filter membrane to obtain a human serum sample; 2) feeding the human serum sample into a chromatographic column, washing by a balancing buffer solution, then eluting by a eluting buffer solution and collecting eluent to obtain the immune globulin IgG solution, the chromatographic column is filled with a combined type bionic chromatography medium, the combined type bionicchromatography medium is prepared from a chromatography matrix and combined type ligand, the chromatography matrix is a hydrophilic porous microsphere with hydroxyl, and a sequence of the combined type ligand is phenylalanine-tyrosine-glutamine-5-aminobenzimidazole; 3) performing utlrafiltration and concentration on the immune globulin IgG solution to obtain the immune globulin IgG. The method can improve an efficiency and a purity for separating the immune globulin IgG from blood.
Owner:ZHEJIANG UNIV
Method for separating human serum albumin by expanded bed adsorption based on mixed mode
ActiveCN106749623AReduce degradationHigh single-step chromatographic puritySerum albuminPeptide preparation methodsSeparation technologyFreeze-drying
The invention discloses a method for adsorbing and separating human serum albumin by expanded bed adsorption based on a mixed mode, which can directly separate and recombine human serum albumin from a yeast fermentation solution, and belongs to the protein separation technology in the biochemical field. The method comprises the following steps: (1) preprocessing a fermentation solution; obtaining the yeast fermentation solution containing the human serum albumin, adding sodium caprylate, and heating and deactivating protease; (2) performing expanded bed adsorption, directly separating the yeast fermentation solution by using an expanded bed filled with a mixed-mode adsorption agent, and collecting elution peaks; and (3) desalting and drying: desalting a collected solution, freeze drying to obtain the human serum albumin with the purity greater than 95 percent. The invention is characterized by developing a novel separation process, so that the high-purity recombined human serum albumin can be directly separated from the yeast fermentation solution. A critical point of the invention lies in adopting the expanded bed adsorption agent based on the mixed mode, cells do not need to be removed from the yeast fermentation solution, the ion strength does not need to be adjusted, the elution condition is moderate, and the method has the characteristics of simple process, high separation efficiency and high yield.
Owner:ZHEJIANG UNIV
Application of modified resin in process of boron removal
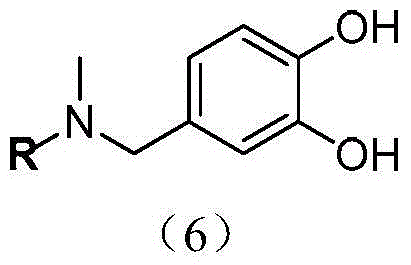
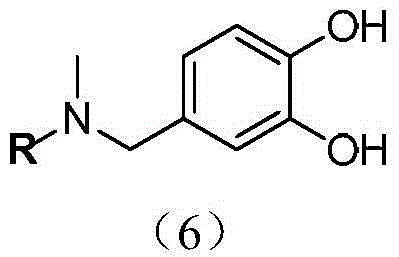
InactiveCN104671322AGood boron removal abilityMild elution conditionsWater/sewage treatment by sorptionElutionBoric acid
The invention discloses an application of modified resin in the process of boron removal. The structure of the modified resin is shown by a formula in the specification, wherein R represents macroporous Merrifield resin with the model number of G20M1254. An experiment proves that the modified resin is relatively good in boron removal capacity in an aqueous solution, and the elution condition is mild, so that the service life of the resin is greatly prolonged. Moreover, the application disclosed by the invention is simple and convenient to operate and easy to implement, is low in equipment requirement, can greatly save the cost and has a practical application value. A hydroxyl radical and a benzene ring of a phenolic compound in the structure of the modified resin are positioned on the same plane, so that o-diphenol can be complexed with boric acid smoothly. The boron removal capacity of the modified resin can reach 6.16mg / g, and the environmental pollution caused by the modified resin can be reduced.
Owner:TIANJIN UNIV
Preparation and application of flag peptide tagged recombinant protein immunoaffinity purification and enrichment column
InactiveCN103111093AStrong specificityEasy to identifySolid sorbent liquid separationPeptide preparation methodsPolyclonal antibodiesFLAG peptide
The invention provides a D-tag tagged recombinant protein immunoaffinity purification and enrichment column capable of efficiently and specifically enriching flag peptide tagged protein and a preparation method and application thereof. The provided immunoaffinity purification and enrichment column comprises an activated agarose filler coupled with an immunoaffinity-purified D-tag specific polyclonal antibody and a plastic column loaded with the immunoaffinity filler, wherein the D-tag specific polyclonal antibody coupled with the immunoaffinity filler is extracted by an immunoaffinity method. The immunoaffinity purification and enrichment column is prepared by loading the immunoaffinity filler into the special plastic column; and the immunoaffinity purification and enrichment column is high in efficiency and strong in specificity while enriching D-tag tagged recombinant protein, is mild in diluting conditions, can be repeatedly utilized and is an ideal material for separating, purifying and enriching the D-tag tagged recombinant protein.
Owner:NANNING LANGUANG BLUE LIGHT BIOTECH
Post-extraction process for swine alpha-interferon
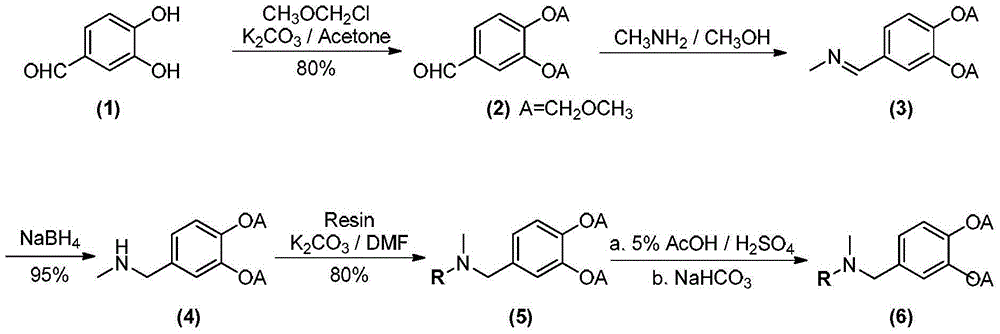
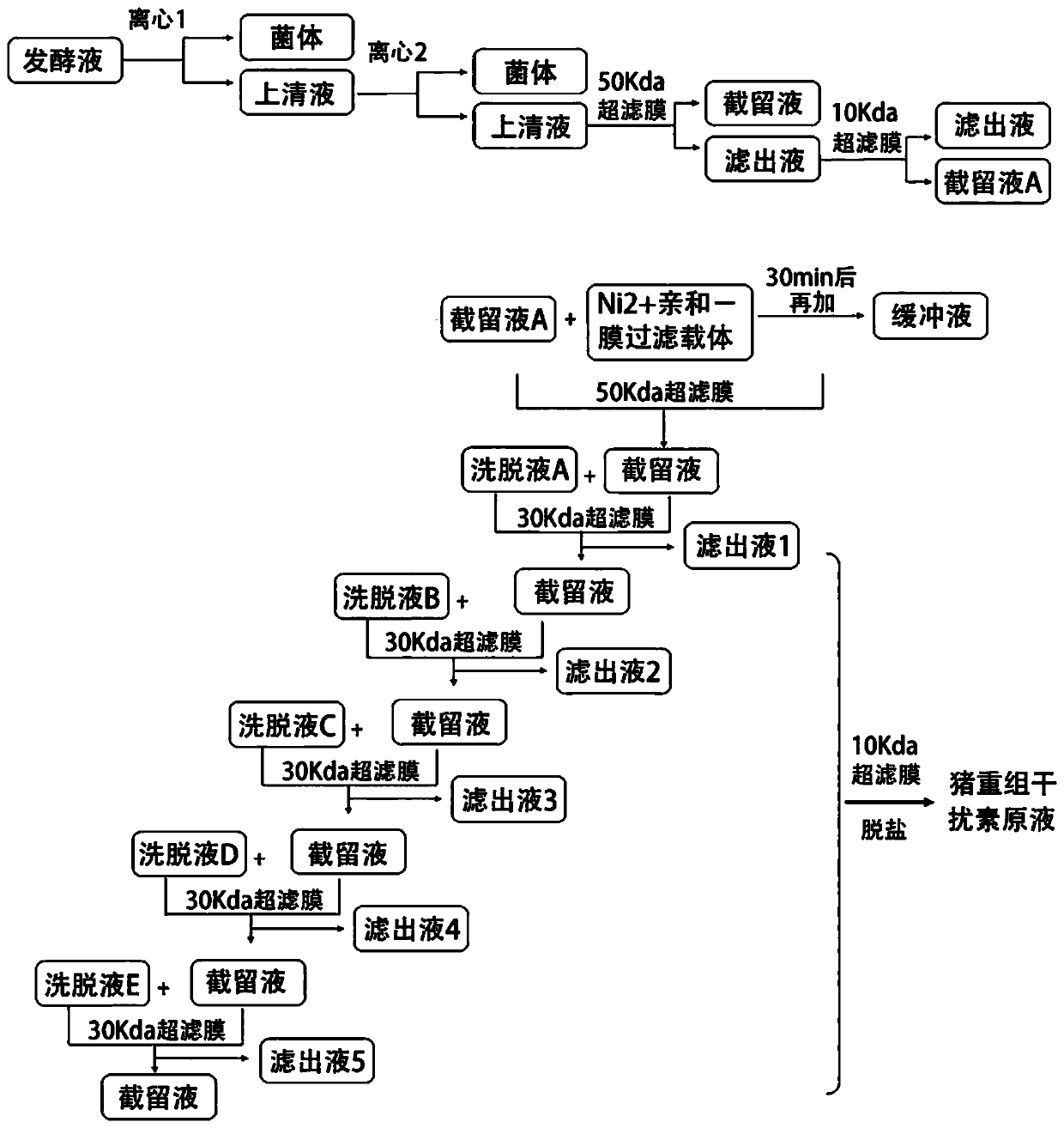
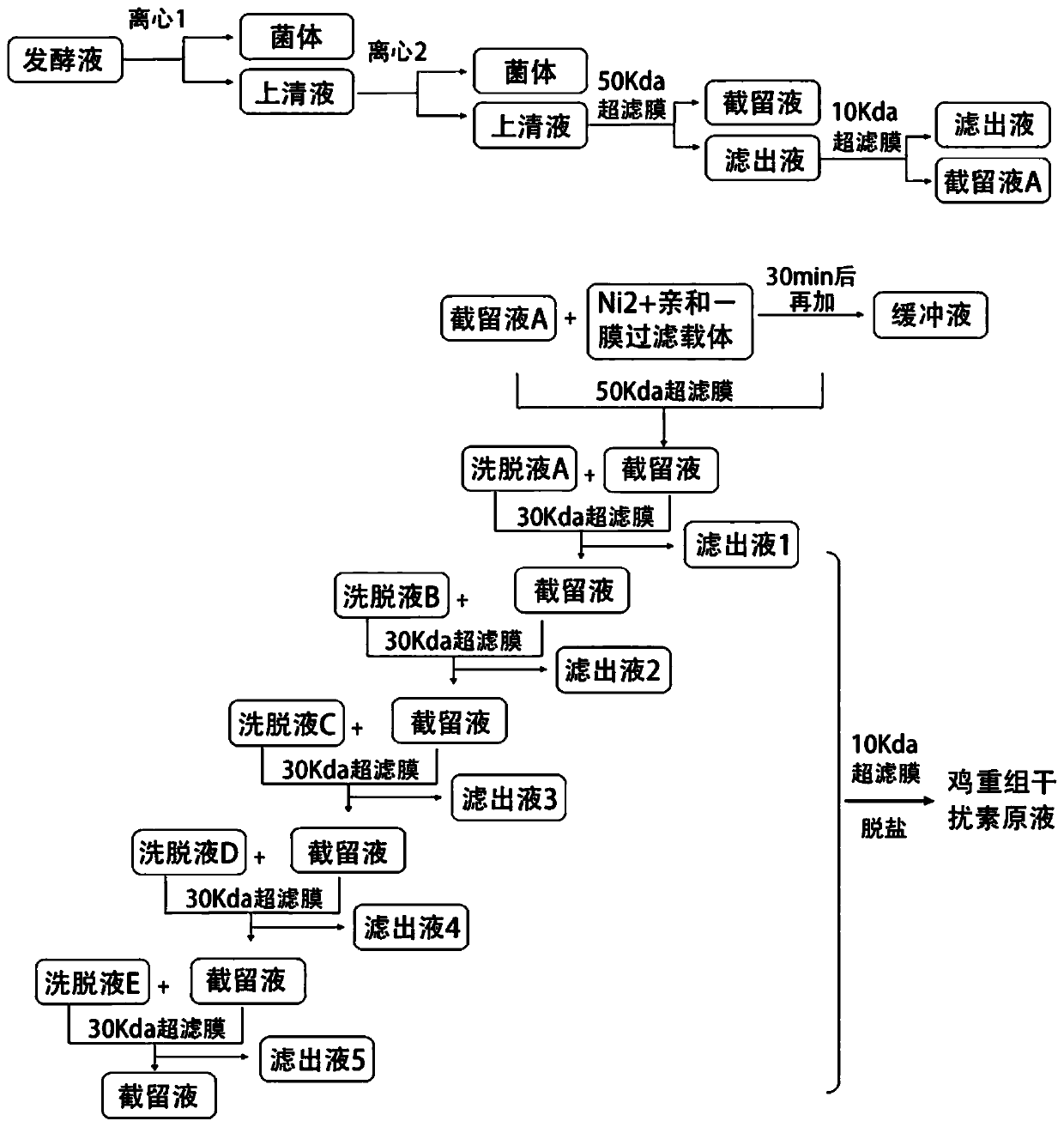
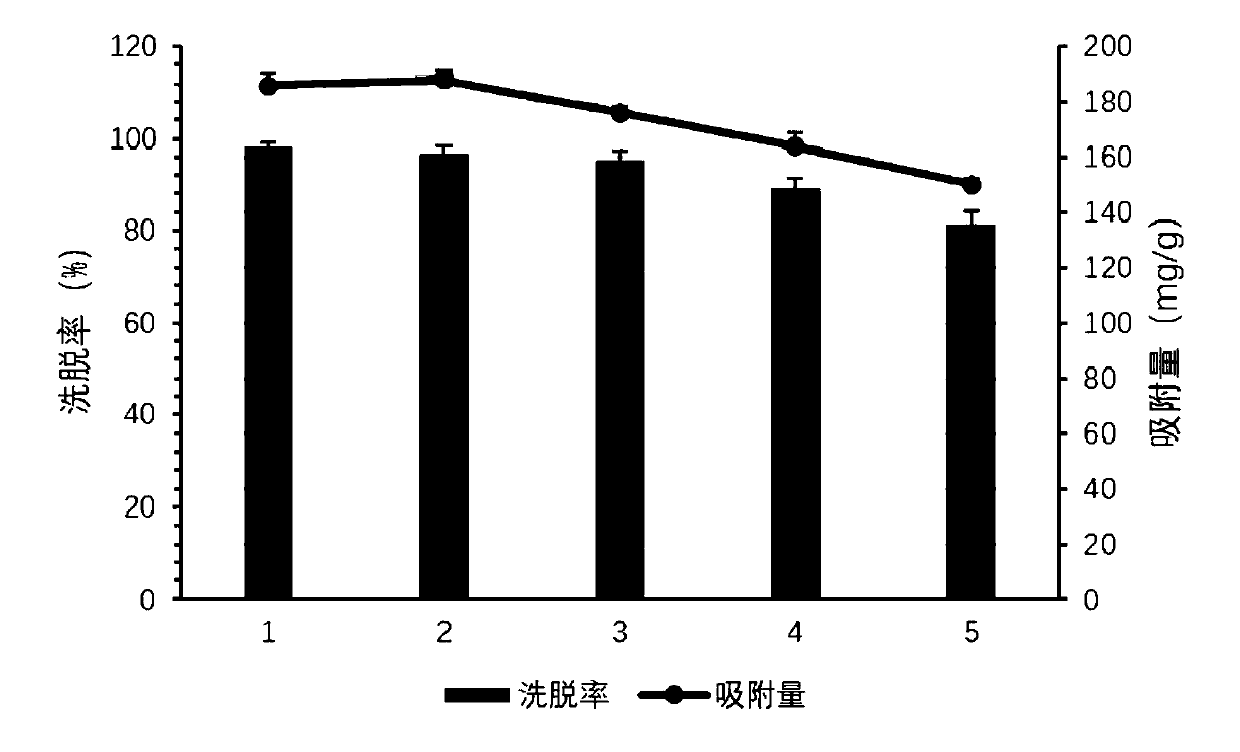
InactiveCN110606884ALow costHigh purityPeptide preparation methodsInterferonsCentrifugationPhosphate
The invention relates to a post-extraction process for swine alpha-interferon and belongs to the technical field of production of drugs for livestock and poultry. The process comprises the steps: S1.subjecting swine alpha-interferon containing fermentation broth to centrifugation, and reserving supernatant; S2. filtering the supernatant through a 50KDa ceramic membrane, then, filtering filter liquor through a 10KDa ceramic membrane, and collecting trapped fluid; S3. adding an equal volume of Ni<2+>-IDA-glucan vector and a NaCl solution into the trapped fluid obtained in the step S2, and carrying out an adsorption reaction, so as to obtain a reaction solution; S4. adding an equal volume of phosphate buffer solution into the reaction solution obtained in the step S3, carrying out uniform mixing, then, carrying out filtering through a 50KDa ceramic membrane, and collecting trapped fluid; S5. adding an eluent into the trapped fluid obtained in the step S4, then, carrying out filtering through a 30KDa ceramic membrane, and collecting filter liquor; and S6. filtering the filter liquor obtained in the step S5 through a 10KDa ceramic membrane to remove salts, and collecting trapped fluid,thereby obtaining the swine alpha-interferon. According to the process, the yield is 62% or more, the purity is 98% or more, and the active yield is 100%.
Owner:山东仙普爱瑞科技股份有限公司
Preparation and Application of Hexahistidine-tagged Protein Immunoaffinity Purification Enrichment Column
InactiveCN103111094BStrong specificityEasy to identifyPreparing sample for investigationSolid sorbent liquid separationPolyclonal antibodiesPolyhistidine-tag
The invention provides an His-tagged recombinant protein immunoaffinity purification and enrichment column capable of efficiently and specifically enriching hexahistidine-tagged protein and a preparation method and application thereof. The provided immunoaffinity purification and enrichment column comprises an activated agarose filler coupled with an immunoaffinity-purified His-specific polyclonal antibody and a plastic column loaded with the immunoaffinity filler, wherein the His-specific polyclonal antibody coupled with the immunoaffinity filler is extracted by an immunoaffinity method. The immunoaffinity purification and enrichment column is prepared by loading the immunoaffinity filler into the special plastic column; and the immunoaffinity purification and enrichment column is high in efficiency and strong in specificity while enriching His-tagged recombinant protein, is mild in diluting conditions, can be repeatedly utilized and is an ideal material for separating, purifying and enriching the His-tagged recombinant protein.
Owner:NANNING LANGUANG BLUE LIGHT BIOTECH +1
A performance-improved recombinant Staphylococcus aureus protein a affinity ligand and its construction method
ActiveCN103214563BImprove toleranceMild elution conditionsMicroorganism based processesDepsipeptidesHigh concentrationEscherichia coli
The present invention discloses a performance improved recombination staphylococcus aureus protein A affinity ligand and a construction method thereof. The present invention adopts a molecular biology method. A sequence B of a nature protein A is selected for molecular transformation. A C-terminal of the sequence B is added with two cysteines, so that the protein A can pass through double-locus coupled chromatography matrix to stabilize the connection. Six glycines are added to the end of a second Loop of the sequence B to increase the length and reduce the binding force with an antibody, so that elution conditions are mild. On this basis, resistance performance to high concentration base of the protein A is transformed. Asparagines and phenylalanine at 23rd and 30th positions of the sequence B are respectively replaced by threonine and alanine to obtain a sequence Z with higher alkaline resistance properties. Then, isocaudarner is used for connecting sequence Zs of different numbers head-to-tail in series. The efficient expression system of e. coli is used for overexpression. The expressed recombination protein A is coupled to agarose matrix preparation affinity chromatography fillers and is used for purifying antibodies. Results show that the recombination protein A affinity ligand prepared by the present invention is good in elution performance and alkali resistance.
Owner:嘉兴千纯生物科技有限公司
Biomimetic monolithic material with affine selectivity similar to that of protein A and preparation method and application thereof
InactiveCN102675602BHigh selectivityEasy to makePeptide preparation methodsImmunoglobulinsEpoxyPhenylboronic acid
The invention discloses a biomimetic monolithic material with the affine selectivity similar to that of a protein A, and a preparation method thereof, wherein the material is capable of purifying, separating and immobilizing antibodies. N, N'-di-(2-aminoethyl) oxamide and 4-mercapto-phenylboronic acid are used as common functional monomers, tri-(2, 3- epoxy group propyl group) isocyanate is used as a monomer, polyethylene glycol is used as a porogenic agent, and reaction is carried out in a mode of in-situ ring-opening polymerization to prepare the biomimetic monolithic material with the affine selectivity similar to that of the protein A. The material can be combined with antibodies specifically, and can be applied to the fields of antibody purification, separation, immobilization and the like. The material has the advantages of high selectivity, low cost, good stability, easiness in elution, reusability and the like, and the immunity affinity and the selectivity of the antibodies are not affected.
Owner:NANJING UNIV
Post-extraction technology of chicken alpha-disruptor
InactiveCN110627893ALow costHigh purityMicroorganism based processesPeptide preparation methodsPhosphateCentrifugation
The invention relates to a post-extraction technology of a chicken alpha-disruptor, and belongs to the technical field of production of medicines for livestock. The technology comprises the steps of S1, performing centrifugation on fermentation liquid containing the chicken alpha-disruptor, and reserving supernatant; S2, filtering the supernatant with a 50KDa ceramic membrane, filtering filtrate through a 10KDa ceramic membrane, and collecting trapped liquid; S3, adding a Cu<2+>-IDA-glucan carrier and a NaCl solution, with the same volume to the trapped liquid in the step S2, and performing anadsorption reaction to obtain reaction liquid; S4, adding a phosphate buffer solution with the same volume to the reaction liquid in the step S3, performing uniform mixing, performing filtering withthe 50KDa ceramic membrane, and collecting trapped liquid; S5, adding eluent to the trapped liquid in the step S4, then performing filtering through the 30KDa ceramic membrane, and collecting filtrate; and S6, filtering the filtrate in the step S5 through the 10KDa ceramic membrane for desalinating, and collecting trapped liquid to obtain the chicken alpha-disruptor. The yield is 61% or above, thepurity is 98% or above, and the activity yield is 96.73% or above.
Owner:山东仙普爱瑞科技股份有限公司
A method for isolating immunoglobulin IgG in human serum
ActiveCN108059673BHigh purity of IgGHigh puritySerum immunoglobulinsPeptide preparation methodsPhenylalanine+TyrosineSerum samples
Owner:ZHEJIANG UNIV
A Mixed Mode Based Expanded Bed Adsorption Method for Separation of Human Serum Albumin
ActiveCN106749623BReduce degradationHigh single-step chromatographic puritySerum albuminPeptide preparation methodsSeparation technologyFreeze-drying
The invention discloses a method for adsorbing and separating human serum albumin by expanded bed adsorption based on a mixed mode, which can directly separate and recombine human serum albumin from a yeast fermentation solution, and belongs to the protein separation technology in the biochemical field. The method comprises the following steps: (1) preprocessing a fermentation solution; obtaining the yeast fermentation solution containing the human serum albumin, adding sodium caprylate, and heating and deactivating protease; (2) performing expanded bed adsorption, directly separating the yeast fermentation solution by using an expanded bed filled with a mixed-mode adsorption agent, and collecting elution peaks; and (3) desalting and drying: desalting a collected solution, freeze drying to obtain the human serum albumin with the purity greater than 95 percent. The invention is characterized by developing a novel separation process, so that the high-purity recombined human serum albumin can be directly separated from the yeast fermentation solution. A critical point of the invention lies in adopting the expanded bed adsorption agent based on the mixed mode, cells do not need to be removed from the yeast fermentation solution, the ion strength does not need to be adjusted, the elution condition is moderate, and the method has the characteristics of simple process, high separation efficiency and high yield.
Owner:ZHEJIANG UNIV
Preparation and application of enrichment column for immunoaffinity purification of recombinant protein tagged with flag peptide
InactiveCN103111093BStrong specificityEasy to identifySolid sorbent liquid separationPeptide preparation methodsProtein tagPolyclonal antibodies
The invention provides a D-tag tagged recombinant protein immunoaffinity purification and enrichment column capable of efficiently and specifically enriching flag peptide tagged protein and a preparation method and application thereof. The provided immunoaffinity purification and enrichment column comprises an activated agarose filler coupled with an immunoaffinity-purified D-tag specific polyclonal antibody and a plastic column loaded with the immunoaffinity filler, wherein the D-tag specific polyclonal antibody coupled with the immunoaffinity filler is extracted by an immunoaffinity method. The immunoaffinity purification and enrichment column is prepared by loading the immunoaffinity filler into the special plastic column; and the immunoaffinity purification and enrichment column is high in efficiency and strong in specificity while enriching D-tag tagged recombinant protein, is mild in diluting conditions, can be repeatedly utilized and is an ideal material for separating, purifying and enriching the D-tag tagged recombinant protein.
Owner:NANNING LANGUANG BLUE LIGHT BIOTECH
A nitrogen-heterocyclic organic polymer monolithic material and its preparation and application
ActiveCN111333776BImprove bindingLow priceOther chemical processesSerum immunoglobulinsPolymer scienceAntiendomysial antibodies
The invention belongs to the field of biomedical materials, and discloses a nitrogen-heterocyclic organic polymer integral material as well as its preparation and application. The preparation method is as follows: mixing nitrogen-heterocyclic monomer compounds, cross-linking agents, porogens and initiators, then ultrasonically dissolving, degassing, and pouring them into a container; polymerization reaction to obtain the nitrogen-heterocyclic organic polymer integral material. The nitrogen-heterocyclic organic polymer integral material obtained in the present invention can be used for capturing monoclonal antibody drugs in complex biological samples. It has the advantages of simple and rapid preparation strategy, low cost, good permeability, stable physical and chemical properties, long service life, less non-specific adsorption, mild elution conditions and will not damage the conformation and activity of antibody proteins.
Owner:JINAN UNIVERSITY
A mixed-mode chromatographic method for separating human albumin from yeast fermentation broth
ActiveCN107033236BLarge adsorption capacityGood choiceSerum albuminPeptide preparation methodsChromatographic separationFreeze-drying
Owner:ZHEJIANG UNIV
Affinity chromatography medium with tetrapeptide as functional ligand and preparation method thereof
ActiveCN104645949BHigh affinityGood choiceOther chemical processesSolid sorbent liquid separationSodium acetateAcetic anhydride
The invention discloses an affinity chromatography medium employing tetrapeptide as a functional ligand and a preparation method of the affinity chromatography medium. The method comprises the following steps: adding dry matrix and allyl bromide to a dimethyl sulfoxide solution, activating, and reacting activating matrix with N-bromo succinimide; enabling bromo alcoholized matrix to react with hexamethylendiamine to obtain amino activating matrix; sequentially washing with deionized water, absolute ethyl alcohol and anhydrous N,N-dimethyl formamide, adding an N,N-dimethyl formamide solution containing tetrapeptide, 2-(7-azobenzotriazole)-N,N,N',N'-te-tramethyluronium hexafluorophosphate and N,N-diisopropylethylamine, and coupling a tetrapeptide ligand; and putting a medium coupled to tetrapeptide in a mixed liquid of sodium acetate and acetic anhydride to obtain the affinity chromatography medium employing tetrapeptide as the functional ligand. According to the novel chromatography medium developed by the method, a functional group is tetrapeptide composed of tyrosine, phenylalanine, arginine and histidine, and is designed on the basis of a protein A binding site of an antibody Fc segment; the antibody binding selectivity is greatly improved; and the affinity chromatography medium can be applied to efficient separation of an antibody.
Owner:ZHEJIANG UNIV
An affinity biomimetic chromatography medium with tetrapeptide as functional ligand
ActiveCN110180505BLarge adsorption capacityHigh affinityIon-exchange process apparatusOther chemical processesPhenylalanine+TyrosineArginine
The invention discloses an affinity biomimetic chromatography medium with a tetrapeptide as a functional ligand, wherein the affinity biomimetic chromatography medium can be used for antibody separation and purification, and the amino acid sequence of the tetrapeptide is phenylalanine-tyrosine-tryptophan-arginine. The preparation method comprises: activating with allyl bromide, carrying out bromination alcoholization, linking a space arm hexanediamine to a polysaccharide gel, and conjugating with an acetylated phenylalanine-tyrosine-tryptophan-arginine tetrapeptide ligand to obtain the tetrapeptide chromatography medium. According to the present invention, the tetrapeptide chromatography medium has good adsorption capacity to antibodies, has high selectivity, can adsorb antibodies under weak alkaline (pH value of 8.0) conditions, can be eluted under weak acidic (pH value of 5.0) conditions, has mild separation conditions, and can be used for isolating immunoglobulins from human serum and isolating monoclonal antibodies in cell culture.
Owner:ZHEJIANG UNIV
Method for preparing microspheric PGDT separating medium with two kinds of pore forms
InactiveCN1233438CImprove hydrophilicityEasy to makeIon-exchange process apparatusOther chemical processesMicrosphereSolid acid
The invention discloses a preparation method of a microsphere PGDT separation medium with two types of pores. The method is to mix the monomer, cross-linking agent, liquid porogen, and initiator first, then add the solid porogen and mix evenly, and form microspheres through suspension polymerization, extract the microspheres with absolute ethanol, and pickle , and dry, two types of pore-type separation media can be obtained. It is characterized in that: the solid porogen is calcium carbonate, and the dosage accounts for 10-3% of the volume content of the reaction mixture; the volume ratio of the solid porogen to the liquid porogen is 0.5-1; the dosage of the porogen accounts for 3% of the volume content of the reaction mixture. 20-60%; the amount ratio of the two cross-linking agents is 0.5-2, and the ratio of the amount of cross-linking agent to the amount of monomer is 0.3-0.6; under normal pressure, the polymerization temperature is controlled at 65-85 between ℃. The separation medium prepared by the method can be used for the separation of biomacromolecules such as proteins after modification, has large adsorption capacity and mild elution conditions, and has good application prospects in large-scale preparative separation of biomacromolecules.
Owner:TIANJIN UNIV
A kind of biomimetic small peptide ligand-based affinity-enriched monolithic material and its preparation and application
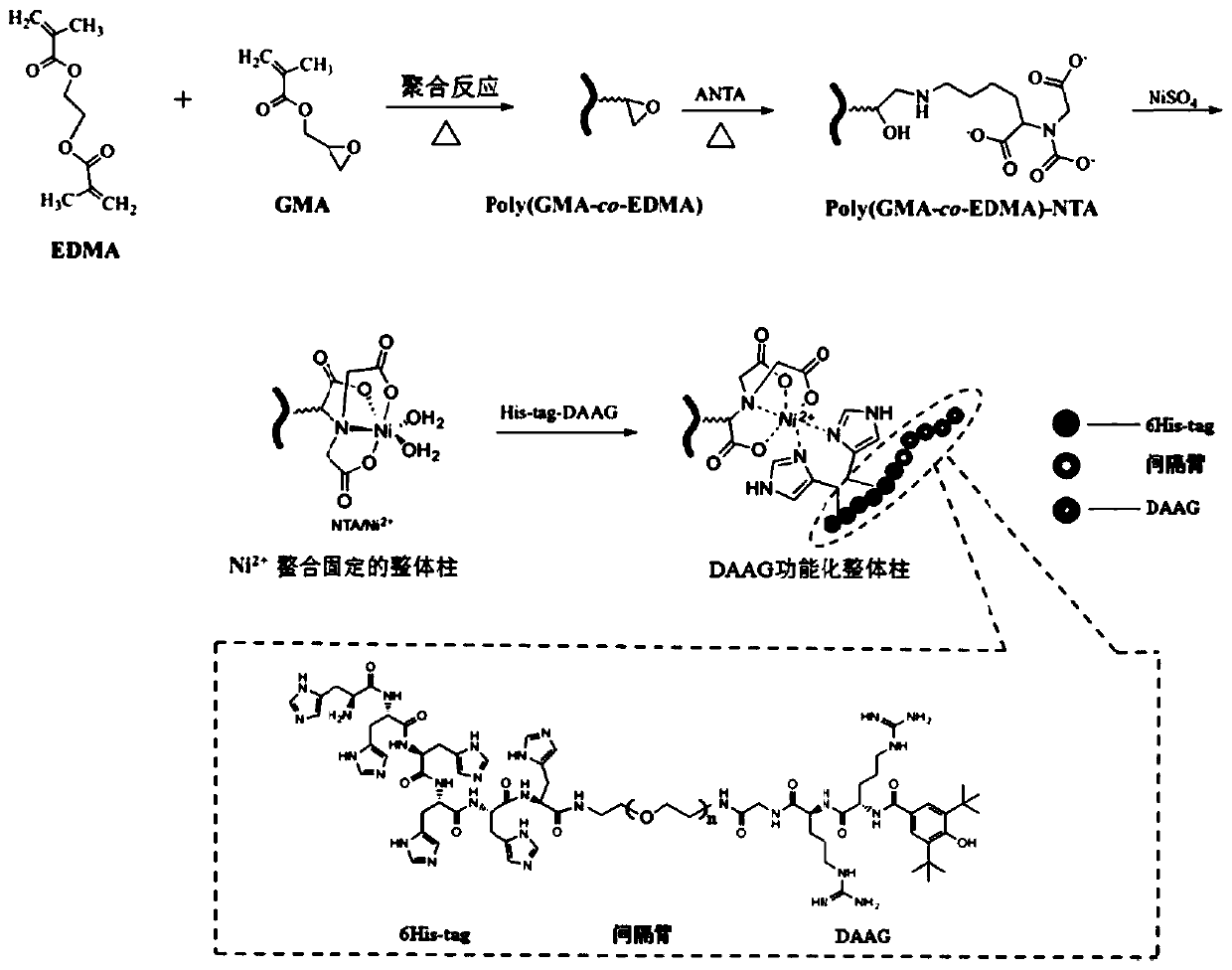
ActiveCN108043365BLow priceChemically stableComponent separationOther chemical processesProtein targetImmunogenicity
The invention belongs to the field of affinity enrichment integral materials and discloses an affinity enrichment integral material based on biomimetic small peptide ligands, preparation and an application. A substrate integral material is prepared from monomer GMA, a crosslinking agent, a pore-foaming agent and an initiator through a mixed reaction; then a metal chelator solution is poured into the substrate material, a heating reaction is performed, then a metal inorganic salt solution is poured for a reaction, so that metal ions are chelated and immobilized to the substrate material, then ahistidine-tagged small peptide ligand solution is poured for a reaction, and the affinity enrichment integral material based on the biomimetic small peptide ligands is obtained. Small peptides are taken as affinity ligands of the integral material, compared with the commonly used biomacromolecular ligands such as protein A / G, antigens or target proteins and the like, the small peptides have the advantages of being low in cost, stable in chemical property, free of immunogenicity and long in service life and containing no biological impurities, and conformation of an antibody protein cannot bedamaged due to quite mild elution conditions.
Owner:JINAN UNIVERSITY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com