A nitrogen-heterocyclic organic polymer monolithic material and its preparation and application
A monolithic material, nitrogen heterocycle technology, applied in the field of biomedical materials, can solve the problems of technical stability and reusability, no small molecule ligand investigation, increase the complexity of the preparation process, etc., and achieve a simple and fast preparation strategy. , low cost, low price effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] The functional monomer 1-vinylimidazole (VIM), cross-linking agent EDMA, porogen (composed of n-propanol, n-decyl alcohol and deionized water) and initiator AIBN were formulated into a polymerization reaction mixed solution according to an optimized ratio, and ultrasonically After dissolving and degassing, it was poured into a 100 μm capillary tube pretreated with γ-MAPs, and reacted in a 60°C water bath for 1.5 hours; the unreacted solution was washed away with methanol to obtain a PVIM organic polymer monolithic chromatographic column. Wherein the mass ratio of monomer VIM and porogen is 22:78, the mass ratio of monomer VIM and crosslinking agent EDMA is 50:50, and the mass ratio of porogen n-propanol, n-decyl alcohol and deionized water is 52.9 :11.8:35.3, the quality of initiator AIBN is 1% of monomer VIM.
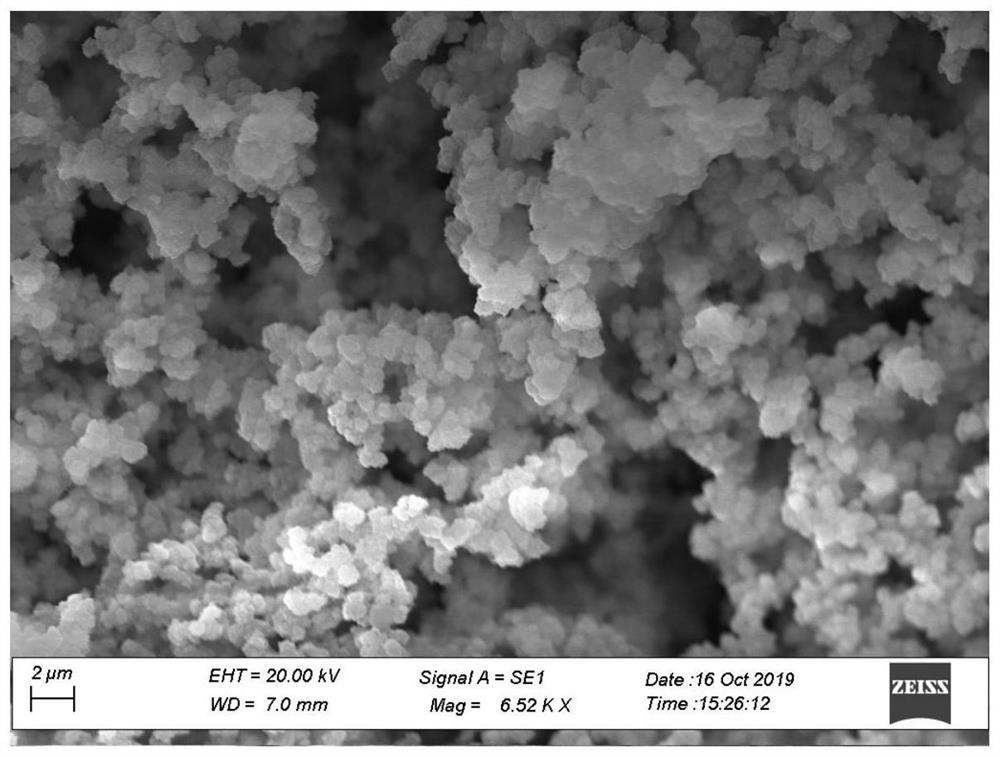
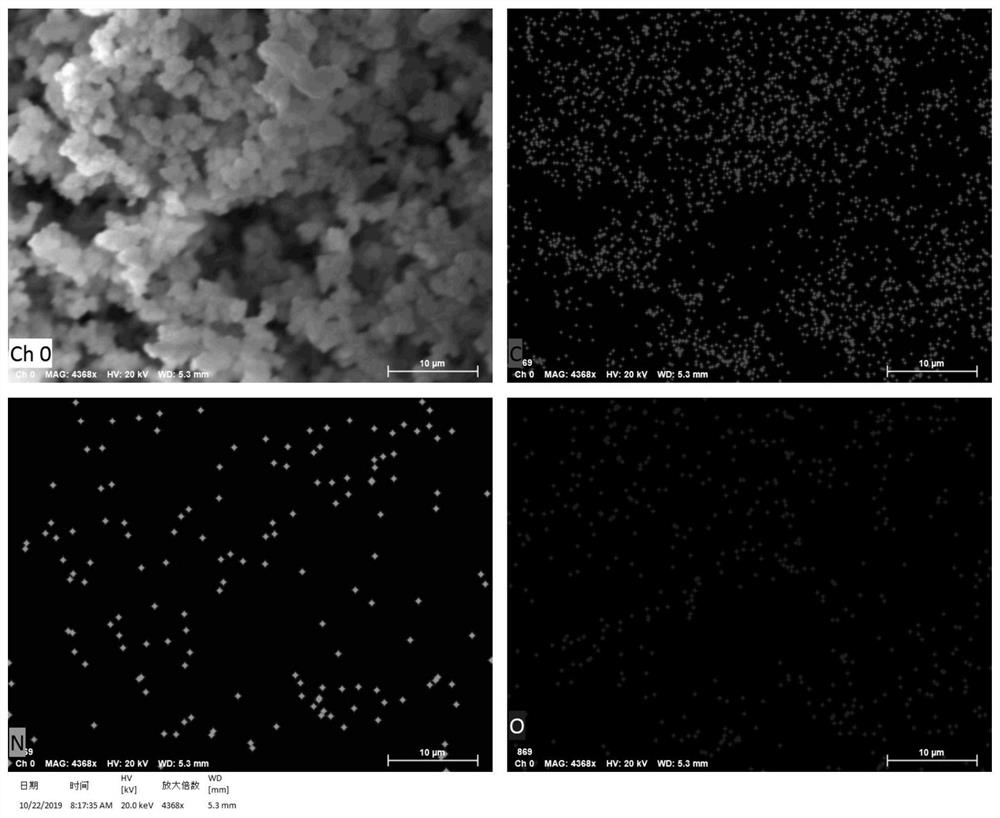
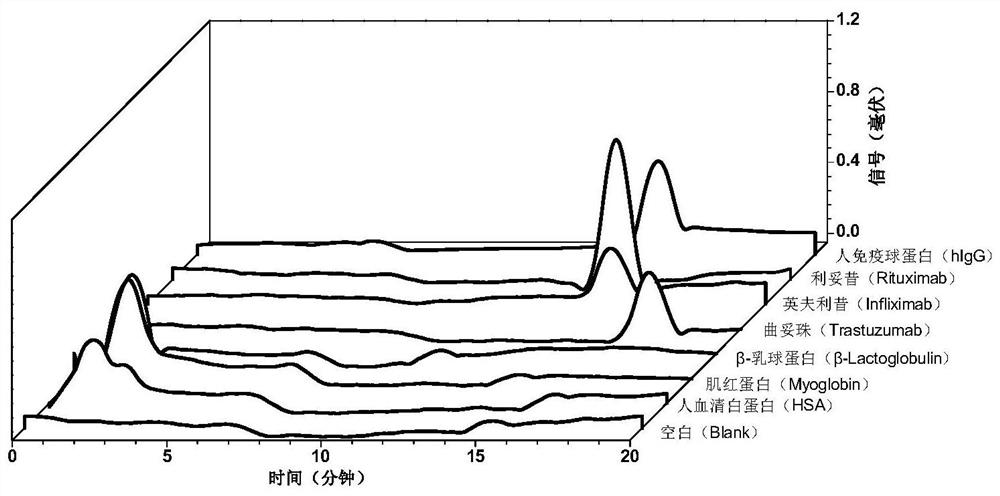
[0049] The scanning electron microscope (SEM) and the energy distribution surface scanning analysis diagram (Elemental mapping) of the internal morphology of th...
Embodiment 2
[0051] The functional monomer 1-vinylimidazole (VIM), cross-linking agent EDMA, porogen (composed of n-propanol, n-decyl alcohol and deionized water) and initiator AIBN were formulated into a polymerization reaction mixed solution according to an optimized ratio, and ultrasonically After dissolving and degassing, pour it into a 200 μL sealed pipette tip, and react in a water bath at 60°C for 1.5 hours; after the reaction is completed, unseal the tip of the pipette, connect the tip of the pipette to the syringe, and rinse it off with methanol The unreacted solution was obtained as a PVIM organic polymer monolithic chromatographic column. Wherein the mass ratio of monomer VIM and porogen is 22:78, the mass ratio of monomer VIM and crosslinking agent EDMA is 50:50, and the mass ratio of porogen n-propanol, n-decyl alcohol and deionized water is 52.9 :11.8:35.3, the quality of initiator AIBN is 1% of monomer VIM.
Embodiment 3
[0053] The functional monomer 1-vinylimidazole (VIM), cross-linking agent EDMA, porogen (composed of n-propanol, n-decyl alcohol and deionized water) and initiator AIBN were formulated into a polymerization reaction mixed solution according to an optimized ratio, and ultrasonically After dissolving and degassing, pour it into a 200 μL sealed pipette tip, and react in a water bath at 60°C for 1.5 hours; after the reaction is completed, unseal the tip of the pipette, connect the tip of the pipette to the syringe, and rinse it off with methanol The unreacted solution was obtained as a PVIM organic polymer monolithic chromatographic column. Wherein the mass ratio of monomer VIM and porogen is 27:73, the mass ratio of monomer VIM and crosslinking agent EDMA is 50:50, and the mass ratio of porogen n-propanol, n-decyl alcohol and deionized water is 52.9 :11.8:35.3, the quality of initiator AIBN is 1% of monomer VIM.
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com