Biomimetic monolithic material with affine selectivity similar to that of protein A and preparation method and application thereof
An integrated material and protein technology, applied in the field of separation, immobilization, and antibody purification, can solve the problems of high price, poor selectivity, and high price of protein A or protein G, and achieve a simple preparation process, mild elution conditions, and easy washing. Fast take-off effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Example 1: Preparation of bulk protein A-like affinity-selective biomimetic monolithic material
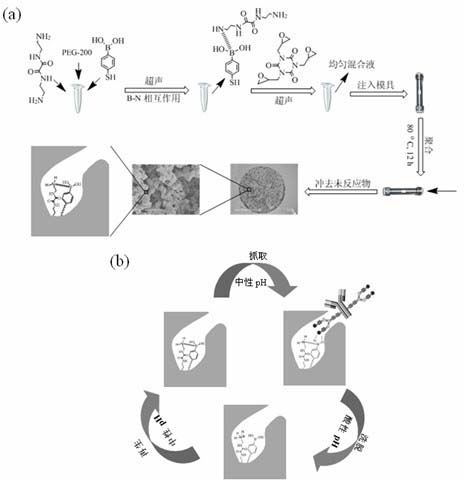
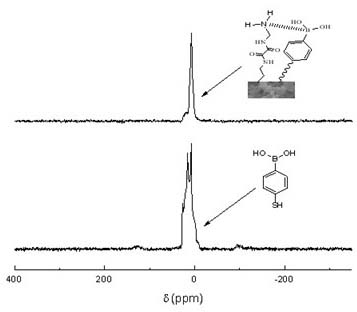
[0029] (1) Take a suitable size centrifuge tube, dissolve 69mg of p-mercaptophenylboronic acid and 61mg of N,N'-bis(2-aminoethyl)oxalamide in 1.1g of PEG-200 solution, vortex, Mix evenly by ultrasonication, then add 240 mg of tris(2,3-epoxypropyl) isocyanate, vortex, ultrasonicate until uniform, seal, and put it in a constant temperature water bath at 80°C for 12 hours.
[0030](2) Then take out the whole material, cut it into small pieces, put it into a Soxhlet extractor, add methanol at a temperature of 110°C, and extract for 24 hours to remove unreacted monomers and cross-linking agents.
[0031] (3) Put the above materials in a vacuum drying oven and dry them at 100°C for 12 hours to obtain a protein A-like affinity-selective biomimetic monolithic material.
[0032]
Embodiment 2
[0033] Embodiment 2: the preparation of the biomimetic monolithic capillary column of protein A affinity selectivity
[0034] Biomimetic monolithic materials with protein A-like affinity and selectivity were synthesized in capillaries with different inner diameters (such as 25 μm, 75 μm, 100 μm, 150 μm, and 250 μm):
[0035] (1) For the pretreatment of the capillary, first rinse the capillary empty column with 0.1M NaOH solution for 1 hour, then rinse the capillary with deionized water until the pH value of the effluent liquid is 7.0, then rinse the capillary with 0.1M HCl solution for 2 hours, and then use The capillary was rinsed with ionized water until the pH of the effluent liquid was 7.0, and then the capillary was rinsed with methanol solution for 30 minutes and dried with nitrogen. Inject a mixture of methanol and methacryloxypropyl-trimethoxysilane at a volume ratio of 1:1 into the capillary. React at a temperature of 20°C to 70°C for 5-24 hours. Then rinse with met...
Embodiment 3
[0041] Example 3: Preparation of biomimetic monolithic material with protein A-like affinity and selectivity in conventional chromatographic columns
[0042] Preparation of protein A-like affinity-selective biomimetic monolithic materials in conventional HPLC columns with different inner diameters:
[0043] (1) To clean the conventional HPLC column, first immerse the conventional HPLC column in deionized water, clean it ultrasonically for 1 hour, then replace it with methanol for ultrasonic cleaning for 1 hour, and dry it in an oven for 2 hours.
[0044] (2) Dissolve 115 mg of p-mercaptophenylboronic acid and 101 mg of N,N'-bis(2-aminoethyl)oxalamide in 1.845 g of PEG-200 solution, and then add 400 mg of tris(2 , 3-epoxypropyl) isocyanate, vortex, and sonicate to obtain a uniformly mixed solution.
[0045] (3) Carefully and slowly add the mixed solution prepared above into the cleaned HPLC conventional column with a pipette gun.
[0046] (4) Seal both ends of the chromatogr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com