Method for preparation of activated carbon nano-iron composite material from kandelia candel leaf
A technology for preparing activated carbon and composite materials, which is applied in the direction of nanotechnology, nanotechnology, nanotechnology, etc. for materials and surface science. Economic and environmental benefits, the effect of overcoming easy deactivation and aggregation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
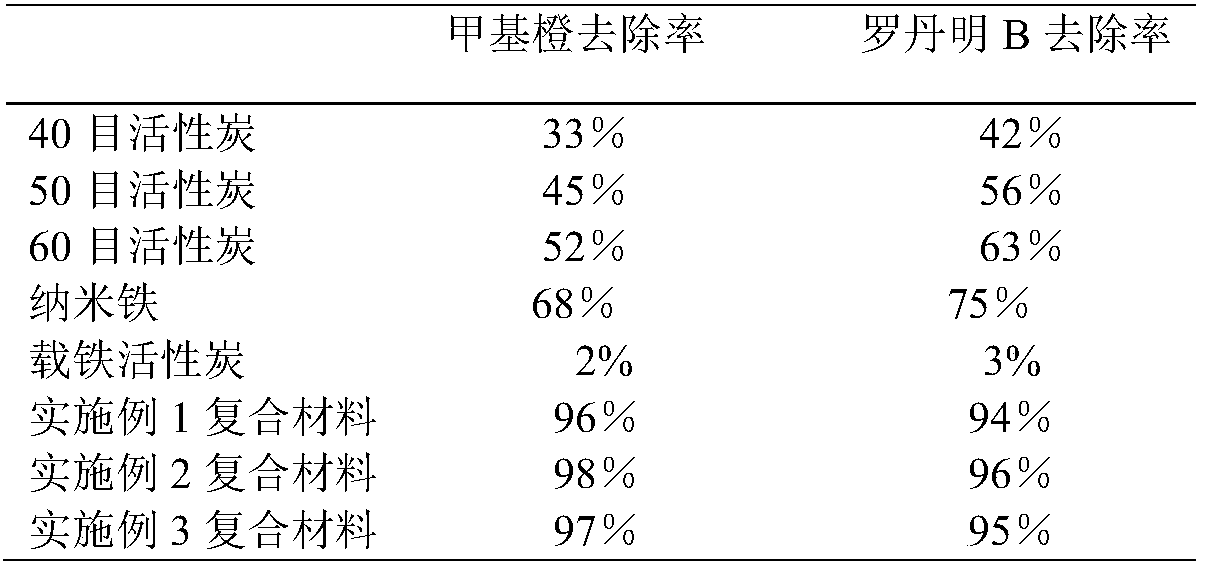
Examples
Embodiment 1
[0027] Using granular activated carbon as a carrier, using polyphenols, flavonoids, caffeine and other biologically active reducing agents contained in the candela leaf extract to greenly synthesize activated carbon nano-iron composite materials, the specific implementation steps are:
[0028] Clean the commercially available granular activated carbon with tap water after passing through a 40-mesh sieve. The cleaned activated carbon was soaked in 2 mL of 0.1 mol / L nitric acid solution per gram for 20 hours, suction filtered and quickly washed with deionized water until the filtrate was neutral, dried in an oven at 105°C for 10 hours, and stored for later use.
[0029] An appropriate amount of ferrous sulfate was dissolved in deionized water to obtain a 0.1 mol / L ferrous sulfate solution. Add 10mL ferrous sulfate solution per gram of pretreated activated carbon, stir magnetically for 2 hours, filter with suction and wash quickly with deionized water until no ferrous ions can be...
Embodiment 2
[0033] Clean the commercially available granular activated carbon with tap water after passing through a 50-mesh sieve. The cleaned activated carbon was soaked in 3 mL of 0.15 mol / L nitric acid solution per gram for 22 hours, suction filtered and quickly washed with deionized water until the filtrate was neutral, dried in an oven at 110°C for 12 hours, and stored for later use.
[0034] An appropriate amount of ferrous sulfate was dissolved in deionized water to obtain a 0.15 mol / L ferrous sulfate solution. Add 15mL of ferrous sulfate solution per gram of pretreated activated carbon, stir magnetically for 3 hours, filter with suction and wash quickly with deionized water until no ferrous ions can be detected in the filtrate, and dry in an oven at 110°C for 12 hours to obtain iron-loaded activated carbon. .
[0035] The collected candela leaves were cleaned with tap water, dried in an oven at 110° C. for 22 hours, crushed, and passed through a 100-mesh sieve to obtain candela ...
Embodiment 3
[0038] Clean the commercially available granular activated carbon with tap water after passing through a 60-mesh sieve. The cleaned activated carbon was soaked in 4 mL of 0.2 mol / L nitric acid solution per gram for 24 hours, suction filtered and quickly washed with deionized water until the filtrate was neutral, dried in an oven at 120°C for 14 hours, and stored for later use.
[0039] An appropriate amount of ferrous sulfate was dissolved in deionized water to obtain a 0.2 mol / L ferrous sulfate solution. Add 20mL of ferrous sulfate solution to each gram of pretreated activated carbon, stir magnetically for 4 hours, filter with suction and wash quickly with deionized water until no ferrous ions are detected in the filtrate, and dry in an oven at 120°C for 14 hours to obtain iron-loaded activated carbon. .
[0040] The collected candela leaves were cleaned with tap water, dried in an oven at 120° C. for 24 hours, crushed, and passed through a 100-mesh sieve to obtain candela l...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com