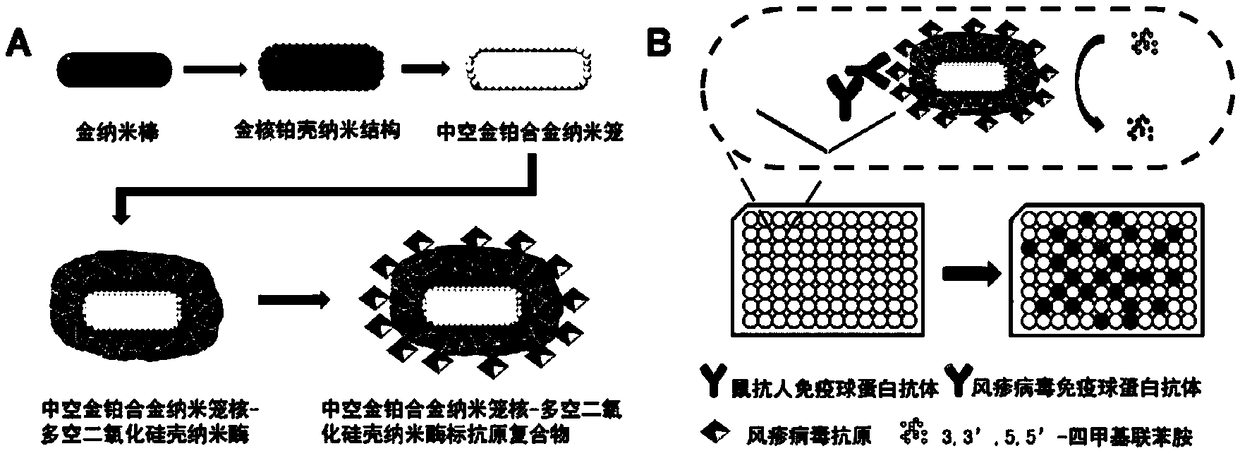
Nano enzyme with nanometer cage core of hollow gold-platinum alloy and shell of porous silicon dioxide, preparation method and application thereof
A gold-platinum alloy and silicon dioxide technology, which is applied in the field of nano-enzymes and gold-platinum alloy core-silica shell nano-enzymes, can solve the problems of low catalytic efficiency of nano-enzymes, and achieves overcoming easy deactivation, extending the working environment and responding wide range of effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
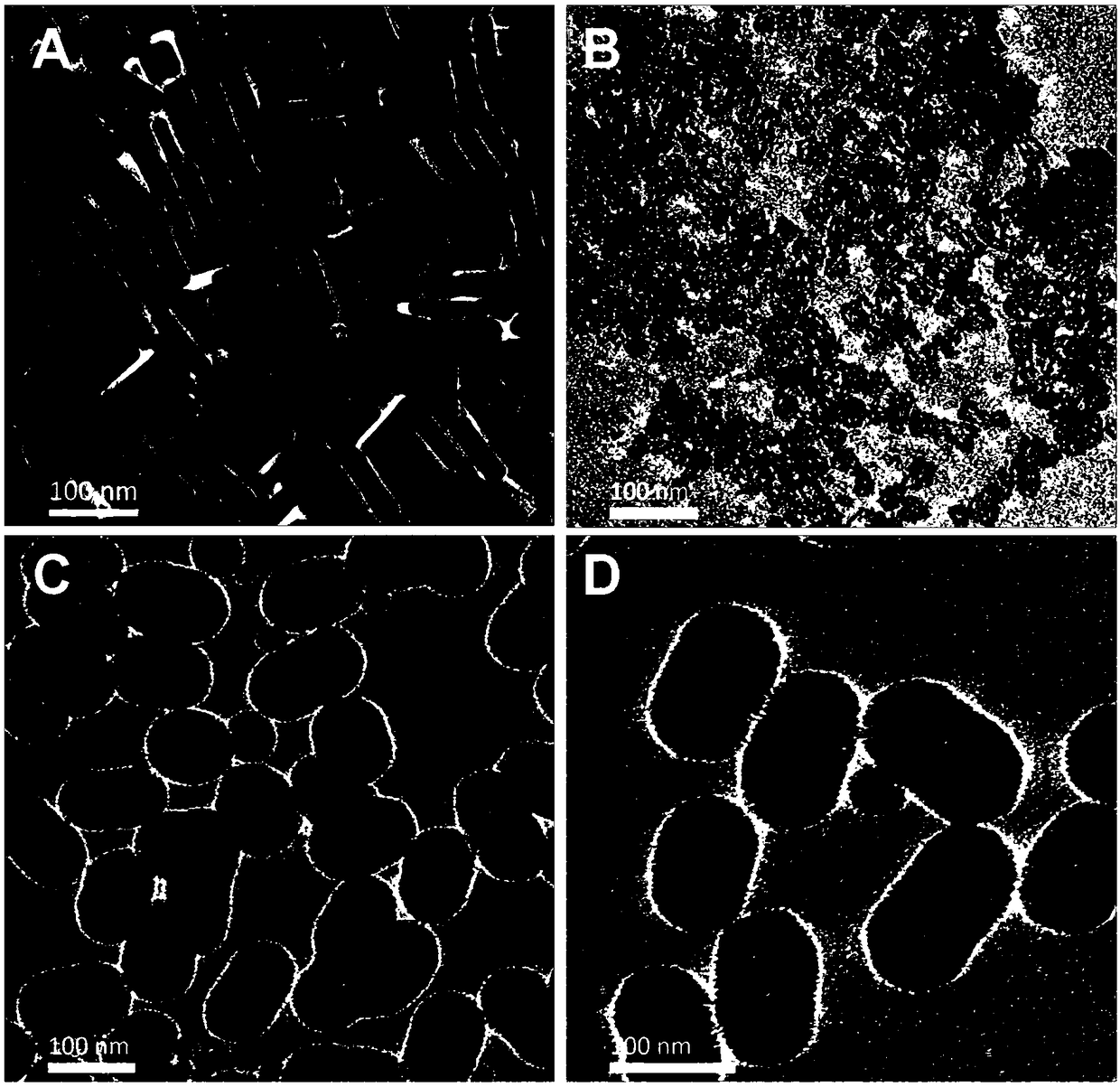
[0033] Example 1 Preparation of hollow gold-platinum alloy nanocage core-porous silica shell nanozyme
[0034] (1) Preparation of gold seeds
[0035] Take 7.5 mL of 0.1 M hexadecyltrimethylammonium bromide aqueous solution, add 100.4 μL of 24.7 mM tetrachloroauric acid aqueous solution to it, mix well and dilute the volume to 9.4 mL, under the condition of magnetic stirring Add 0.6 mL of sodium borohydride aqueous solution with a concentration of 0.01 M (temporarily prepared and placed in ice water before use), stop stirring for 3 min, and let it stand for 2 h to obtain a gold seed solution containing gold seeds. The gold seed solution The gold concentration was 0.25 mM.
[0036] (2) Preparation of gold nanorods
[0037] Take 100 mL of 0.1 M cetyltrimethylammonium bromide aqueous solution, add 2.05 mL of 24.7 mM tetrachloroauric acid aqueous solution, 1 mL of 10 mM silver nitrate aqueous solution and 2 mL of 0.5 M sulfuric acid aqueous solution, after mixing evenly, then ad...
Embodiment 2
[0044] Example 2 Preparation of Nanozyme-labeled Rubella Antigen Complex
[0045] (1) Preparation of hollow gold-platinum alloy nanocage core-porous silica shell nanoenzyme-labeled rubella antigen complex
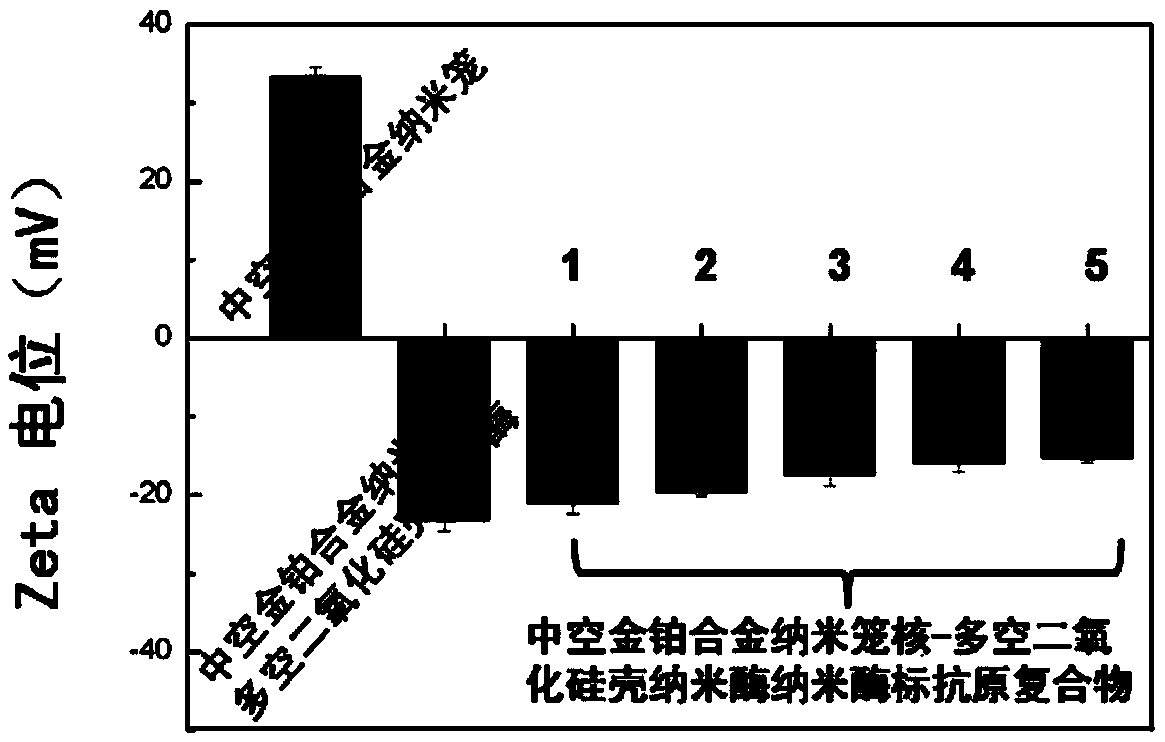
[0046] Take 50 μL of hollow gold-platinum alloy nanocage core-porous silica shell solution (5nM), and disperse it in 450 μL of phosphate buffer solution (0.1M, pH 7) containing 50 μL of 10 mg / mL rubella virus antigen. After mixing evenly, put them in a 37°C incubator, and after incubation for 96 h, centrifuge at 12000 rpm for 5 min to remove free rubella virus antigen molecules. Pour off the supernatant and disperse the precipitate in 100μL phosphate buffer (0.1M, pH 7) for later use. Hollow Au-Pt alloy nanocage prepared by zeta potential characterization, hollow Au-Pt alloy nanocage core-porous silica shell nanozyme and hollow Au-Pt alloy nanocage core-porous silica shell nanoenzyme labeling Rubella antigen Compounds such as image 3 shown. The results showed that the ...
Embodiment 3
[0049] Example 3 Application of nano-enzyme-labeled antigen complex in enzyme-linked immunoassay
[0050] First, 300 μL of mouse anti-artificial immunoglobulin antibody at a concentration of 1 μg / mL was added to each well of a high-affinity 96-well enzyme-labeled microwell plate, and the coating buffer was 0.1 M phosphate buffer (pH=7 ) at 4 °C overnight, then the above-mentioned microplate was washed 5 times with phosphate-Tween buffer, and 300 μL of blocking solution (1.0 wt% bovine albumin) was added to each well to block at 37 °C for 2 After h, the cells were washed 5 times with phosphate-Tween buffer. Then, add 100 μL of a series of serum samples and incubate at 37 °C for 1 h, wash 5 times with phosphate-Tween buffer, then add 50 μL of nano-enzyme-labeled antigen complex and incubate for 0.5 h, so as to capture on the microwell plate The virus immunoglobulin antibody of the sample to be tested forms a primary antibody-secondary antibody-enzyme-labeled antigen complex, an...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com