Stable polypeptide protein-targeted chimeric molecule, preparation method and application thereof
A polypeptide and protein target technology, applied in the field of bioengineering, can solve the problems of poor breast cancer effect and easy drug resistance, and achieve the effect of significant technological progress, good degradation ability and good proliferation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
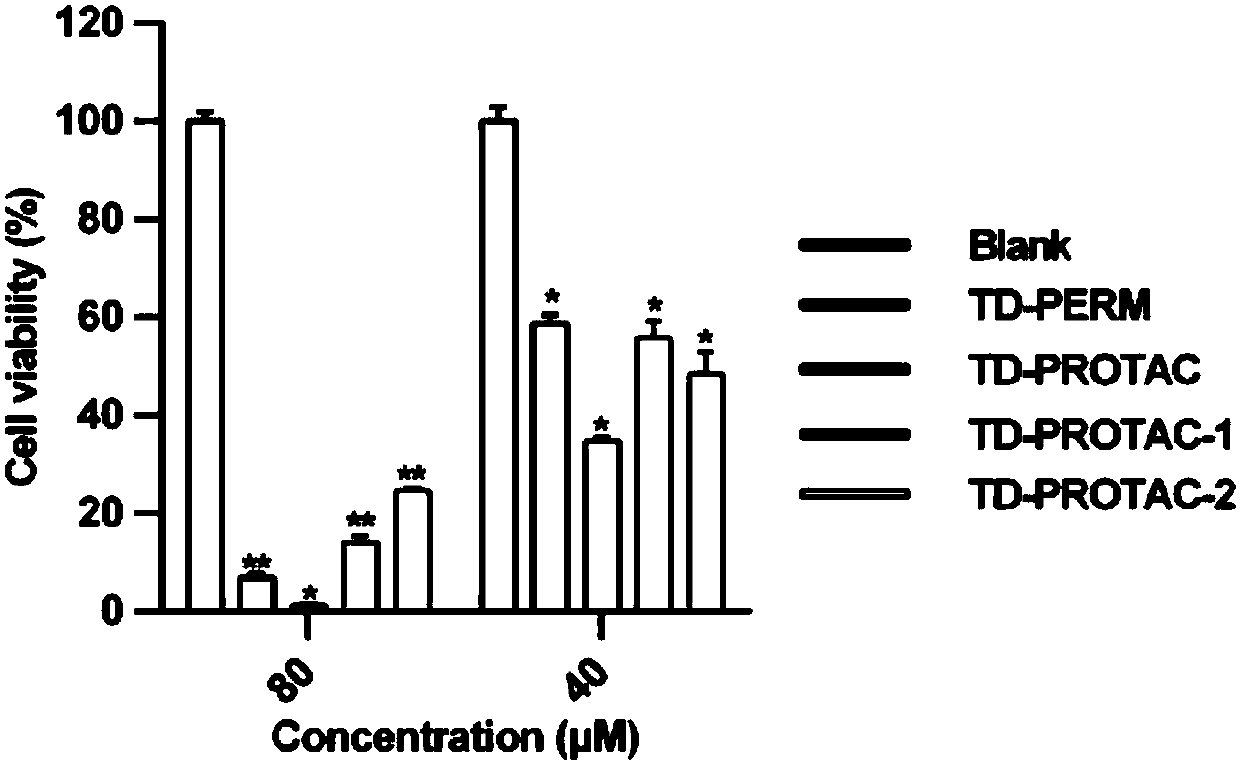
[0038] The present invention adopts protein targeting chimera technology, and a pentapeptide (LAP(OH)YI) that can bind to VonHippel-Lindau (VHL) E3 ubiquitin ligase and a polypeptide (TD) that can target ERα are combined through a linking group. -PERM) to design peptides. Then recruit VHL ubiquitin ligase, and then ubiquitinate ERα, degrade ERα by starting proteasome, and then affect the downstream pathway of ERα, and inhibit the proliferation ability of breast cancer cell lines with high expression of ERα.
[0039] Such as Figure 21 As shown, a stable helical polypeptide (TD-PERM) targeting ERa was obtained by connecting chiral diacid-modified polypeptides at the end side chain-tail end, and then combined with a Von Hippel-Lindau (VHL) E3 ubiquitin ligase A stable polypeptide protein targeting chimera molecule (TD-PROTAC) targeting estrogen receptor alpha obtained by linking the pentapeptide (LAP(OH)YI) to degrade ERα protein through the ubiquitin-proteasome pathway.
[00...
Embodiment 2
[0045] In order to better carry out the following research, the design of the control peptide is necessary. As shown in Table 2, we designed a series of control polypeptides. Our research group previously developed an estrogen receptor regulatory polypeptide stabilized by the terminal aspartic acid strategy and named it TD-PERM, and the pentapeptide that binds to VHL E3 ubiquitin ligase was named HIF. In addition, we scrambled the TD-PERM sequence to obtain the control polypeptide TD-PROTACsc, and mutated the key amino acid residues in the HIF polypeptide that bind to VHL E3 ligase into alanine to obtain the control polypeptide TD-PROTACmut. In addition, we also synthesized a peptide PROTAClinear that did not use the ring-closing strategy.
[0046] Table 2: TD-PROTAC and its control polypeptide sequence (peptides without Tyr residues in the sequence are introduced into Try at the nitrogen end to determine the concentration of the polypeptide. For fluorescence polarization exp...
Embodiment 3
[0048] Preparation and separation and purification steps of the polypeptide of embodiment 3:
[0049] According to the amino acid sequence of solid-phase synthesis of polypeptides, the core steps of preparing the above stable polypeptides are as follows (TD-PROTAC as an example):
[0050]
[0051] The specific operation steps are:
[0052] (1) Polypeptide solid-phase synthesis: Weigh 100 mg Rink amide MBHA resin into a 10 ml peptide tube, add dichloromethane (DCM), and swell with nitrogen gas for 30 min. Add 50% (v / v) morpholine in N,N-dimethylformamide (DMF) solution, blow nitrogen gas for 30 minutes, and remove the Fmoc protecting group. After washing the resin 10 times alternately with DMF and DCM, the prepared (1) Fmoc-Ile-OH (5eq, 0.4M, DMF) solution, 6-chlorobenzotriazole-1,1,3,3-tetramethyl Urea hexafluorophosphate (HCTU) (5eq, 0.38M, DMF) solution and N,N-diisopropylethylamine (DIPEA) (10eq) were mixed well, then added to the resin and blown with nitrogen for 1h. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com