Core-shell catalyst as well as preparation method and application thereof
A catalyst and core-shell technology, applied in the field of core-shell catalysts and their preparation, can solve the problems of low yield of methyl halide and reduction of selectivity of methyl halide
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
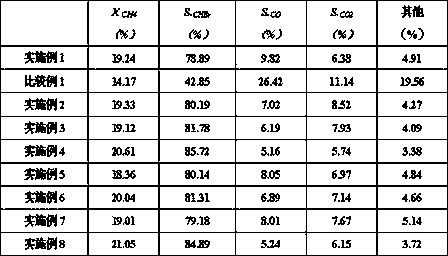
Embodiment 1
[0023] Use aluminum isopropoxide hydrolysis method to prepare aluminum hydroxide slurry: mix water and aluminum isopropoxide at a molar ratio of 120:1, control the hydrolysis temperature at 80℃-85℃, and hydrolyze the aluminum isopropoxide for 1.5 hours before aging The aging temperature is controlled at 90℃-95℃, the aging time is 18h, and an aluminum hydroxide slurry with a solid content of 21.3wt% is obtained.
[0024] Preparation of zinc-loaded composite metal oxide NdCoO with perovskite structure 3-y :Immerse zinc nitrate solution in NdCoO3-y by the equal volume dipping method, and then dry and roast after immersion. The drying time is 2h and the drying temperature is 130°C; the roasting time is 4h and the temperature is 400°C. Composite metal oxide NdCoO with perovskite structure 3-y Spherical, zinc-loaded composite metal oxide NdCoO with perovskite structure 3-y The equivalent diameter is 2mm;
[0025] Spray dipping process: mix an appropriate amount of zirconium sulfate solid...
Embodiment 2
[0029] Use aluminum isopropoxide hydrolysis method to prepare aluminum hydroxide slurry: mix water and aluminum isopropoxide at a molar ratio of 120:1, control the hydrolysis temperature at 80℃-85℃, and hydrolyze the aluminum isopropoxide for 1.5 hours before aging The aging temperature is controlled at 90℃-95℃, the aging time is 18h, and an aluminum hydroxide slurry with a solid content of 21.3wt% is obtained.
[0030] Preparation of zinc-loaded composite metal oxide NdCoO with perovskite structure 3-y :Using equal volume impregnation method in NdCoO 3-y Dipping in zinc sulfate solution, drying and roasting after soaking, the drying time is 3h, the drying temperature is 120°C; the roasting time is 5h, the temperature is 450°C, the zinc-loaded composite metal with perovskite structure is oxidized NdCoO 3-y Spherical, zinc-loaded composite metal oxide NdCoO with perovskite structure 3-y The equivalent diameter is 2mm;
[0031] Spray dipping process: mix an appropriate amount of zirc...
Embodiment 3
[0035] Use aluminum isopropoxide hydrolysis method to prepare aluminum hydroxide slurry: mix water and aluminum isopropoxide at a molar ratio of 120:1, control the hydrolysis temperature at 80℃-85℃, and hydrolyze the aluminum isopropoxide for 1.5 hours before aging The aging temperature is controlled at 90℃-95℃, the aging time is 18h, and an aluminum hydroxide slurry with a solid content of 21.3wt% is obtained.
[0036] Preparation of zinc-loaded composite metal oxide NdCoO with perovskite structure 3-y :Using equal volume impregnation method in NdCoO 3-y Dipped in zinc bromide solution, dried and roasted after soaking. The drying time is 4h and the drying temperature is 100°C; the roasting time is 4h and the temperature is 500°C. The zinc-loaded composite with perovskite structure Metal oxide NdCoO 3-y Spherical, zinc-loaded composite metal oxide NdCoO with perovskite structure 3-y The equivalent diameter is 3mm;
[0037] Spray dipping process: mix an appropriate amount of zirconi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com