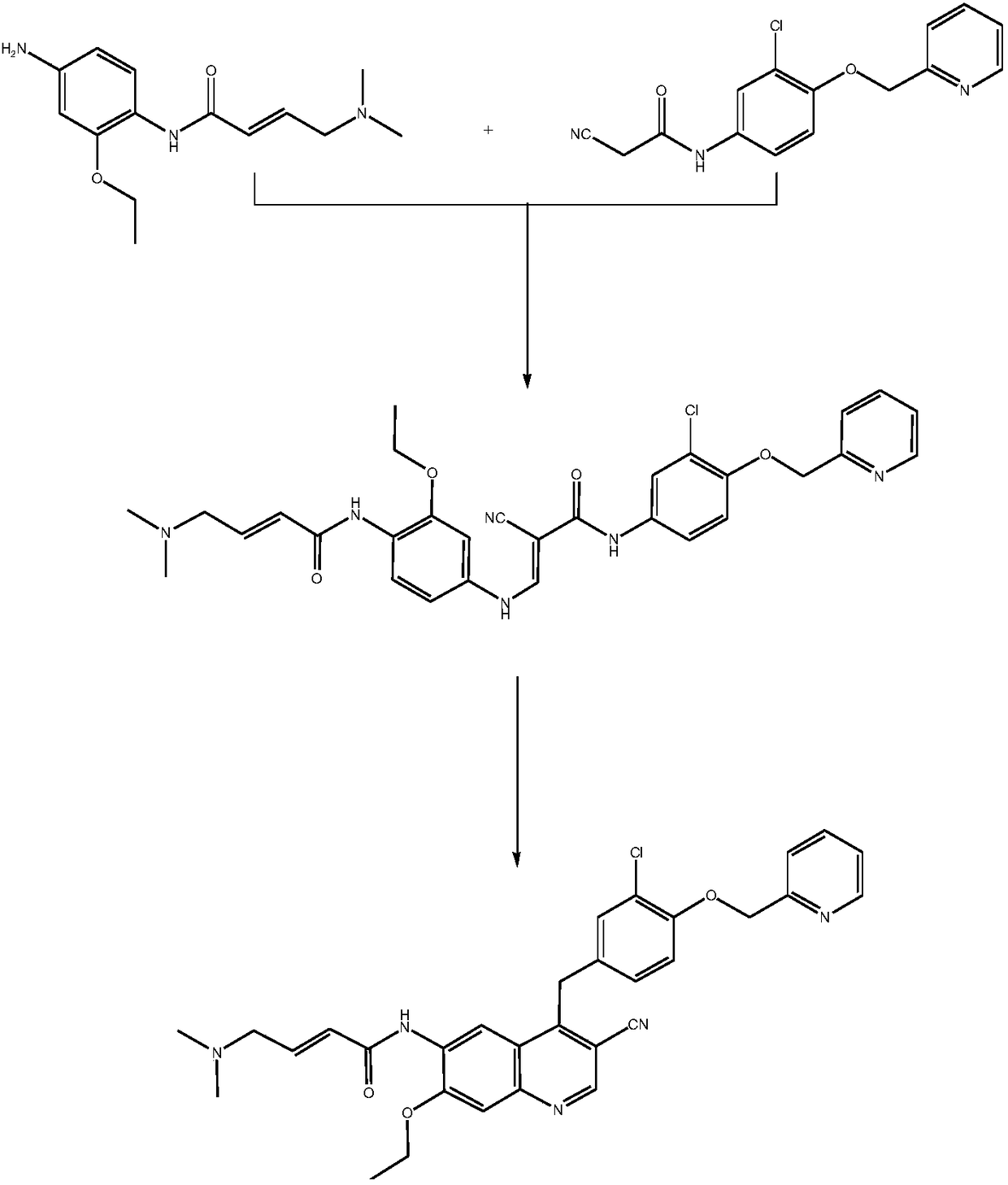
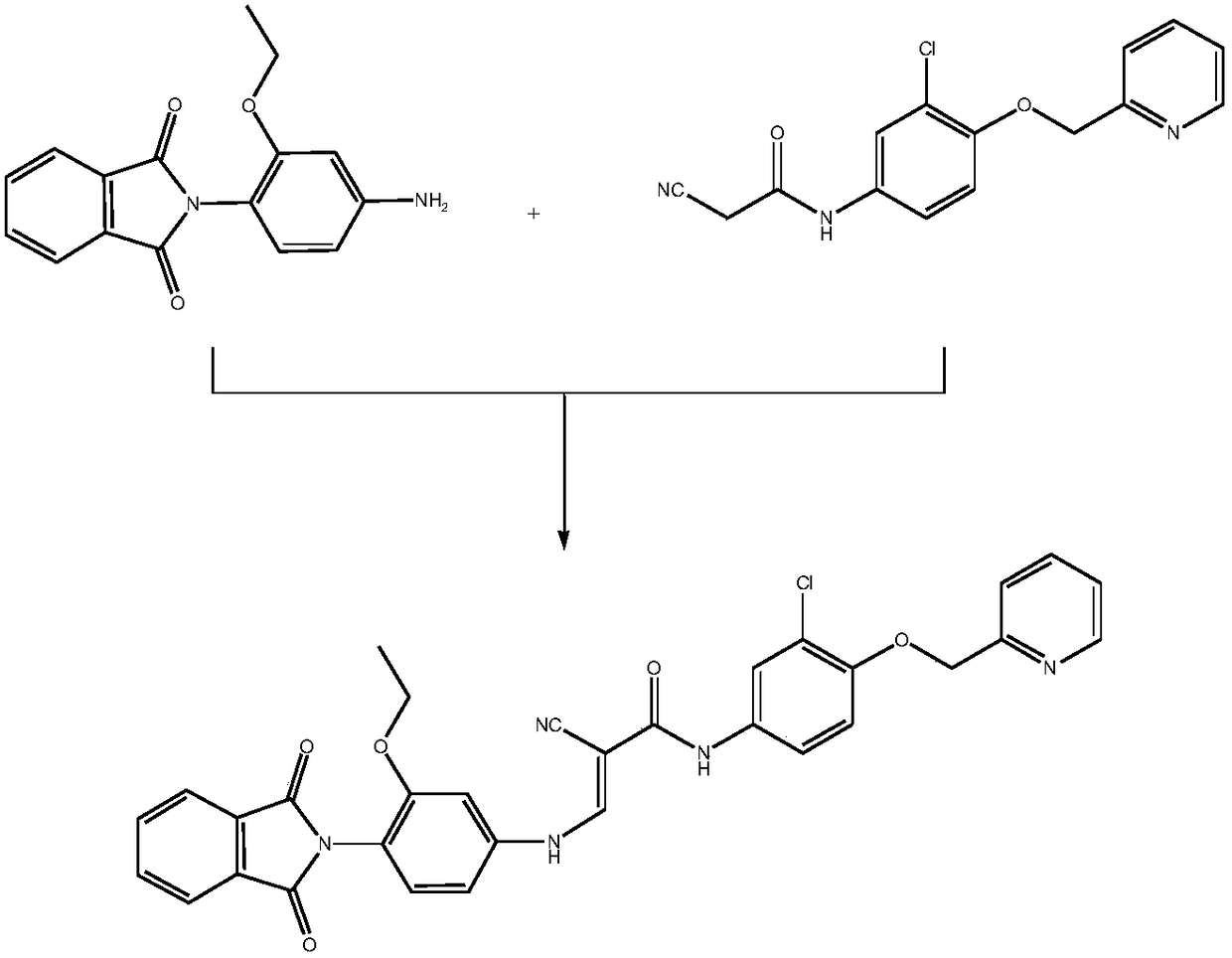
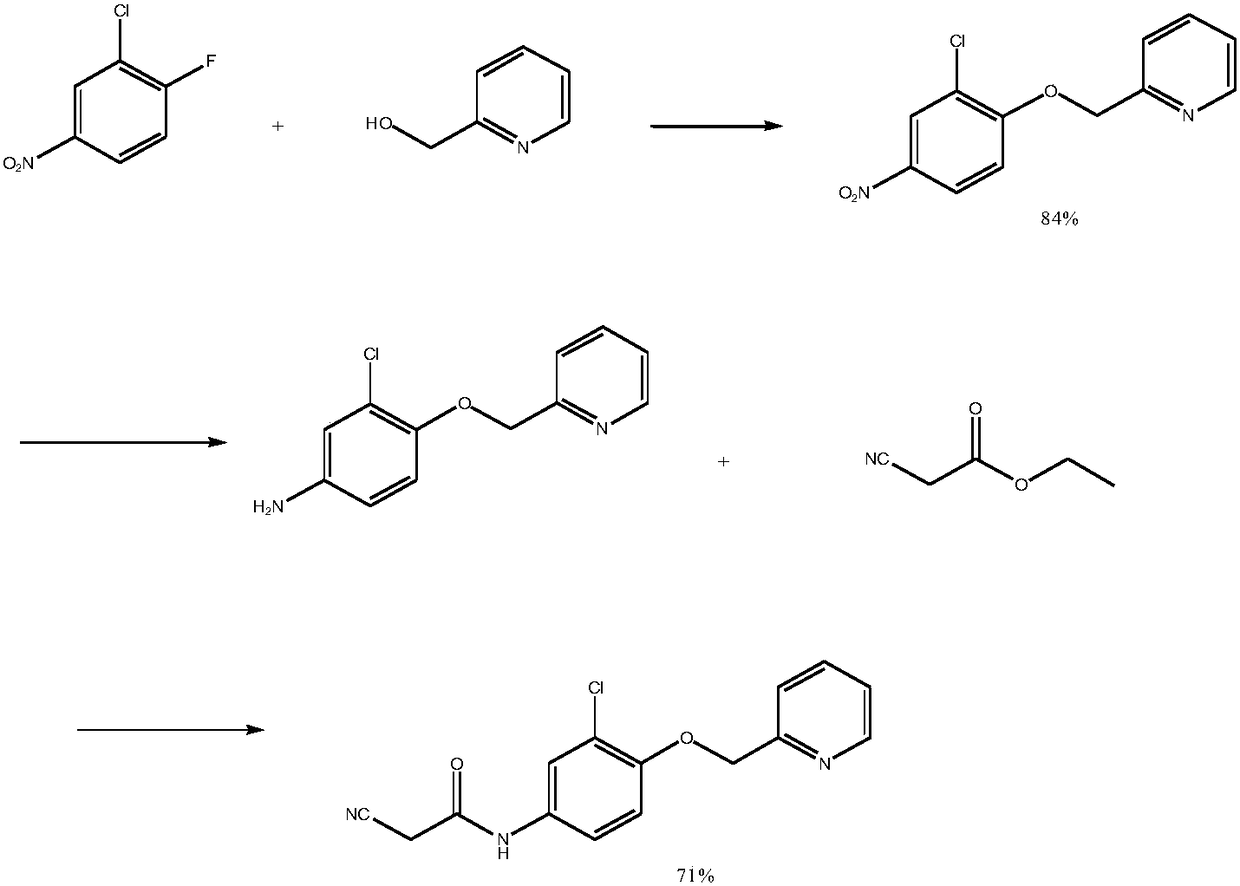
Preparation method of N-(3-chlorine-4-(2-pyridine methoxy)phenyl)-2-cyanoacetamide
A technology of cyanoacetamide and pyridylmethoxyl, which is applied in the field of preparation of N-phenyl)-2-cyanoacetamide, can solve the problems of potential safety hazards, unsuitability for safe production, low yield, etc., and achieve easy Large-scale production, high product yield and simple steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] 1) Synthesis of N-(3-chloro-4-fluorophenyl)-2-cyanoacetamide
[0032] Add 14.6g of 3-chloro-4-fluoroaniline and 10.2g of cyanoacetic acid to 90ml of DMF, stir to dissolve, then add 12.1g of triethylamine, 1-(3-dimethylaminopropyl)-3-ethyl carbon Diimine hydrochloride 25.9g and 1-hydroxybenzotriazole 15.5g, heated to 55°C, stirred for reaction, after the reaction was completed (TLC, dichloromethane: ethyl acetate 1:1), cooled to room temperature, Add 720ml of water, 200ml of dichloromethane to extract, leave the organic layer, add 200ml of water to wash the organic layer, dry the organic layer with anhydrous magnesium sulfate, filter the desiccant, and evaporate the organic layer under reduced pressure to obtain solid N-(3-chloro- 4-fluorophenyl)-2-cyanoacetamide 19.2g, the yield was 90.6%.
[0033] 2) Synthesis of N-(3-chloro-4-(2-pyridinemethoxy)phenyl)-2-cyanoacetamide
[0034] Add 10.5g of 2-pyridinemethanol and 2.8g of lithium hydroxide to 50ml of acetonitrile, add 19.2g...
Embodiment 2
[0036] 1) Synthesis of N-(3-chloro-4-fluorophenyl)-2-cyanoacetamide
[0037] Add 14.6g of 3-chloro-4-fluoroaniline and 10.2g of cyanoacetic acid to DMF 100ml, stir to dissolve, then add 12.3g of triethylamine, 1-(3-dimethylaminopropyl)-3-ethyl carbon 26.2g of diimine hydrochloride and 15.8g of 1-hydroxybenzotriazole, heated to 52°C, stirred for reaction, after the reaction was completed (TLC, dichloromethane: ethyl acetate 1:1), cooled to room temperature, Add 700ml of water and 200ml of dichloromethane for extraction, leave the organic layer, add 200ml of water to wash the organic layer, dry the organic layer with anhydrous magnesium sulfate, filter the desiccant, and evaporate the organic layer under reduced pressure to obtain solid N-(3-chloro- 4-fluorophenyl)-2-cyanoacetamide 19.2g, the yield was 90.6%.
[0038] 2) Synthesis of N-(3-chloro-4-(2-pyridinemethoxy)phenyl)-2-cyanoacetamide
[0039] Add 10.8 g of 2-pyridine methanol and 2.5 g of lithium hydroxide to 60 ml of acetonit...
Embodiment 3
[0041] 1) Synthesis of N-(3-chloro-4-fluorophenyl)-2-cyanoacetamide
[0042] Add 14.6g of 3-chloro-4-fluoroaniline and 10.2g of cyanoacetic acid to 90ml of DMF, stir to dissolve, then add 12.0g of triethylamine, 1-(3-dimethylaminopropyl)-3-ethyl carbon Diimine hydrochloride 25.5g and 1-hydroxybenzotriazole 15.2g, heated to 55°C, stirred for reaction, after the reaction was completed (TLC, dichloromethane: ethyl acetate 1:1), cooled to room temperature, Add 700ml of water and 200ml of dichloromethane for extraction, leave the organic layer, add 200ml of water to wash the organic layer, dry the organic layer with anhydrous magnesium sulfate, filter the desiccant, and evaporate the organic layer under reduced pressure to obtain solid N-(3-chloro- 4-fluorophenyl)-2-cyanoacetamide 19.0 g, yield 89.6%.
[0043] 2) Synthesis of N-(3-chloro-4-(2-pyridinemethoxy)phenyl)-2-cyanoacetamide
[0044] Add 10.5 g of 2-pyridine methanol and 2.5 g of lithium hydroxide to 50 ml of acetonitrile, add 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com