A tuberculosis polypeptide vaccine for preventing and treating tuberculosis and its preparation method and application
A polypeptide vaccine and tuberculosis technology, which is applied to medical preparations without active ingredients, medical preparations containing active ingredients, and pharmaceutical formulas, etc., can solve the problem of unknown tuberculosis vaccines, unknown anti-tuberculosis immune effects, and no tuberculosis antigen polypeptide tuberculosis. And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] In this embodiment, the tuberculosis polypeptide nano-vaccine using chitosan-deoxycholic acid conjugate as a carrier includes chitosan-deoxycholic acid conjugate and tuberculosis polypeptide; the tuberculosis polypeptide is composed of Mycobacterium tuberculosis Rv0180c protein 81 ~100 amino acid fragments, Rv0227c protein 261~280 amino acid fragments and Rv2004c protein 281~300 amino acid fragments.
[0046] The preparation of above-mentioned tuberculosis polypeptide nano-vaccine comprises the following steps:
[0047] (1) Synthesis of three tuberculosis polypeptides
[0048] The 81-100 amino acid fragment of the Mycobacterium tuberculosis Rv0180c protein, the 261-280 amino acid fragment of the Rv0227c protein and the 281-300 amino acid fragment of the Rv2004c protein were synthesized.
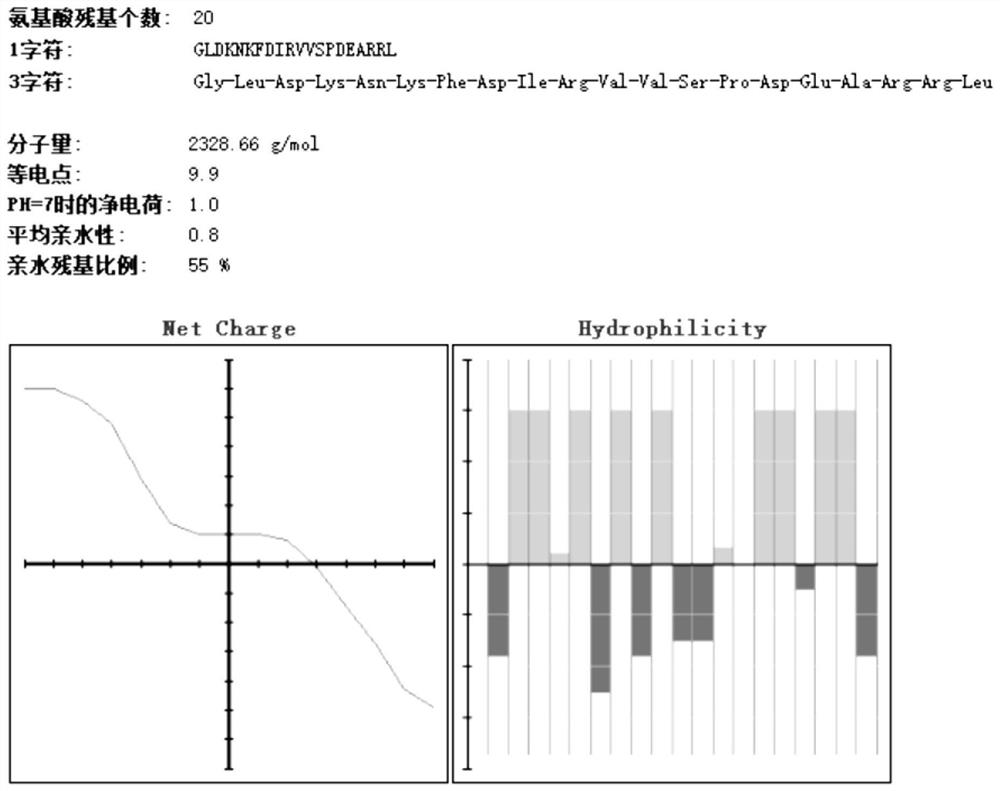
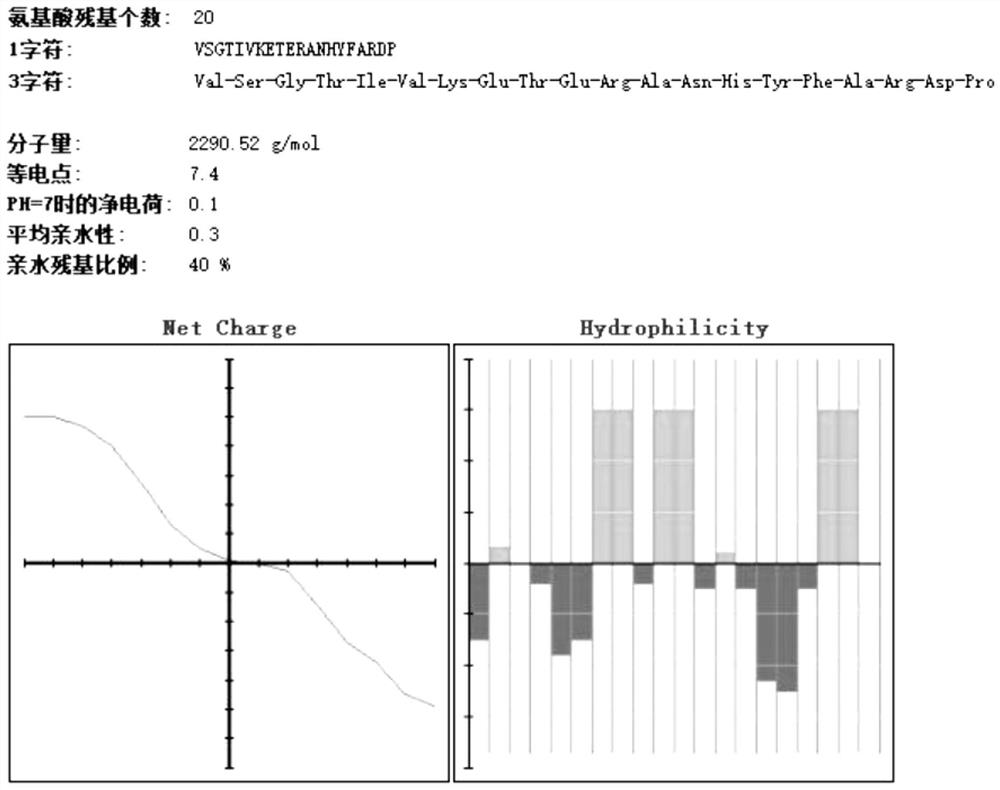
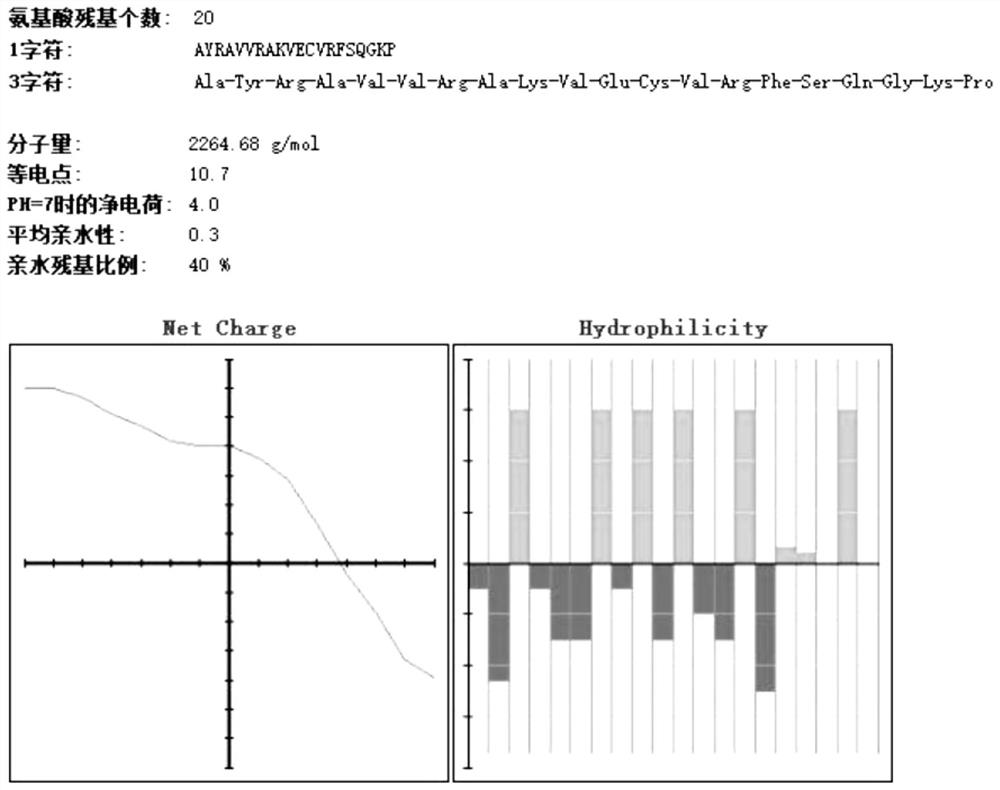
[0049] The basic physicochemical properties of amino acids 81-100 of Rv0180c protein are as follows: figure 1 Shown; The basic physicochemical properties of the 261-280 amino acids o...
Embodiment 2
[0061] In this embodiment, the tuberculosis polypeptide nano-vaccine with chitosan-deoxycholic acid conjugate as carrier includes chitosan-deoxycholic acid conjugate and tuberculosis polypeptide; the tuberculosis polypeptide is composed of Mycobacterium tuberculosis Rv0180c protein The 81-100 amino acid fragment and the 261-280 amino acid fragment of the Rv0227c protein consist of two polypeptides.
[0062] The preparation of above-mentioned tuberculosis polypeptide nano-vaccine comprises the following steps:
[0063] (1) Synthesis of two tuberculosis polypeptides
[0064] Synthetic method is the same as embodiment 1.
[0065] (2) Preparation of chitosan-deoxycholic acid conjugate
[0066] Dissolve 0.15 g of chitosan in 20 mL of 1% aqueous acetic acid, and then dilute with 20 mL of methanol. Add 0.8g of deoxycholic acid and 0.6g of 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDC.HCL) successively to the diluent, stir and react at room temperature 20h. Pou...
Embodiment 3
[0074] In this embodiment, the tuberculosis polypeptide nano-vaccine with chitosan-deoxycholic acid conjugate as a carrier includes chitosan-deoxycholic acid conjugate and tuberculosis polypeptide; the tuberculosis polypeptide is composed of Mycobacterium tuberculosis Rv0227c protein The 261-280 amino acid fragment and the 281-300 amino acid fragment of Rv2004c protein consist of two polypeptides.
[0075] The preparation of above-mentioned tuberculosis polypeptide nano-vaccine comprises the following steps:
[0076] (1) Synthesis of two tuberculosis polypeptides
[0077] Synthetic method is the same as embodiment 1.
[0078] (2) Preparation of chitosan-deoxycholic acid conjugate
[0079] Dissolve 0.25 g of chitosan in 20 mL of 1% aqueous acetic acid, and then dilute with 20 mL of methanol. Add 0.9g of deoxycholic acid and 0.65g of 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDC.HCL) successively to the diluent, stir and react at room temperature 28h. Pou...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com