Reactive yellow dye compound as well as preparation method and application thereof
A technology of dye compound and reactive yellow, which is applied in the field of reactive yellow dye compound and its preparation and application, can solve the problems of good alkali resistance, high reactivity, and poor alkali resistance of covalent bonds, and achieve excellent color fastness, durability Good alkalinity, bright color effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
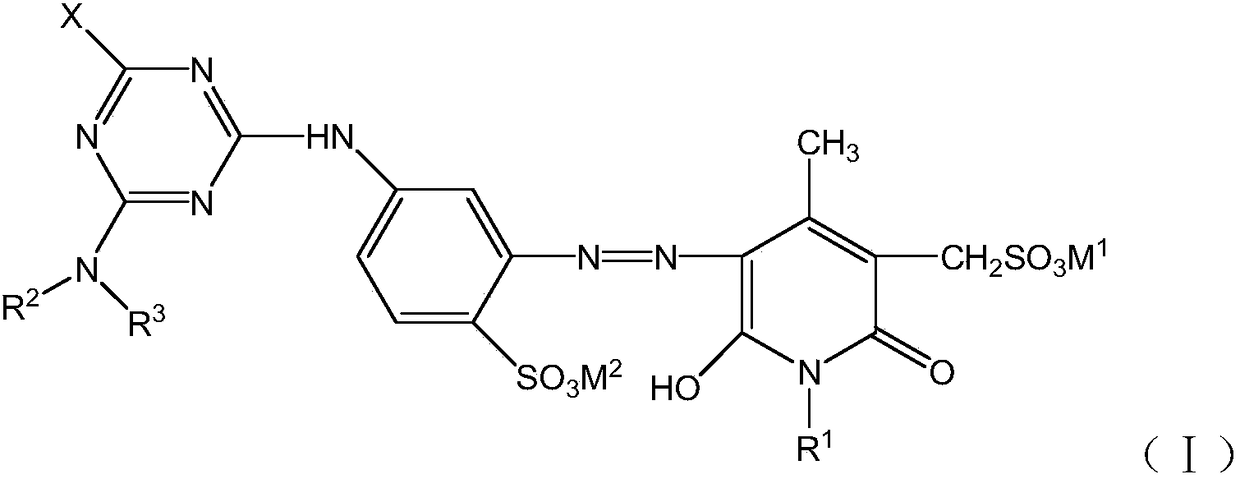
[0037] (1) Primary condensation: 500g of ice water is added to a 1000ml beaker, 20.3g of cyanuric chloride (0.11mol) is put into the beaker for 1 hour, and 20.7g of 4-sulfonic acid-1,3-phenylenediamine (0.11mol) is added after beating ), react for 2 hours at pH 2.0~3.0, temperature 0~5°C, detect the main peak of the liquid phase ≥ 94%, that is, the end of the reaction is reached, and a shrinkage solution is obtained;
[0038] (2) Diazotization: After the shrinkage reaction is completed, add appropriate amount of ice, 31.1g of 31% hydrochloric acid (0.27mol), 7.6g of sodium nitrite (0.11mol), keep the potassium iodide test paper showing blue and no yellow smoke. React at pH 1.0-1.2 and temperature 0-5°C for 2 hours, use sulfamic acid to eliminate excess sodium nitrite until starch potassium iodide test paper does not develop color, and obtain diazonium solution;
[0039] (3) Coupling: Add 27.2g N-ethyl-3-methylsulfonate-4-methyl-6-hydroxyl-2-pyridone (0.11mol) to the diazo solu...
Embodiment 2~15
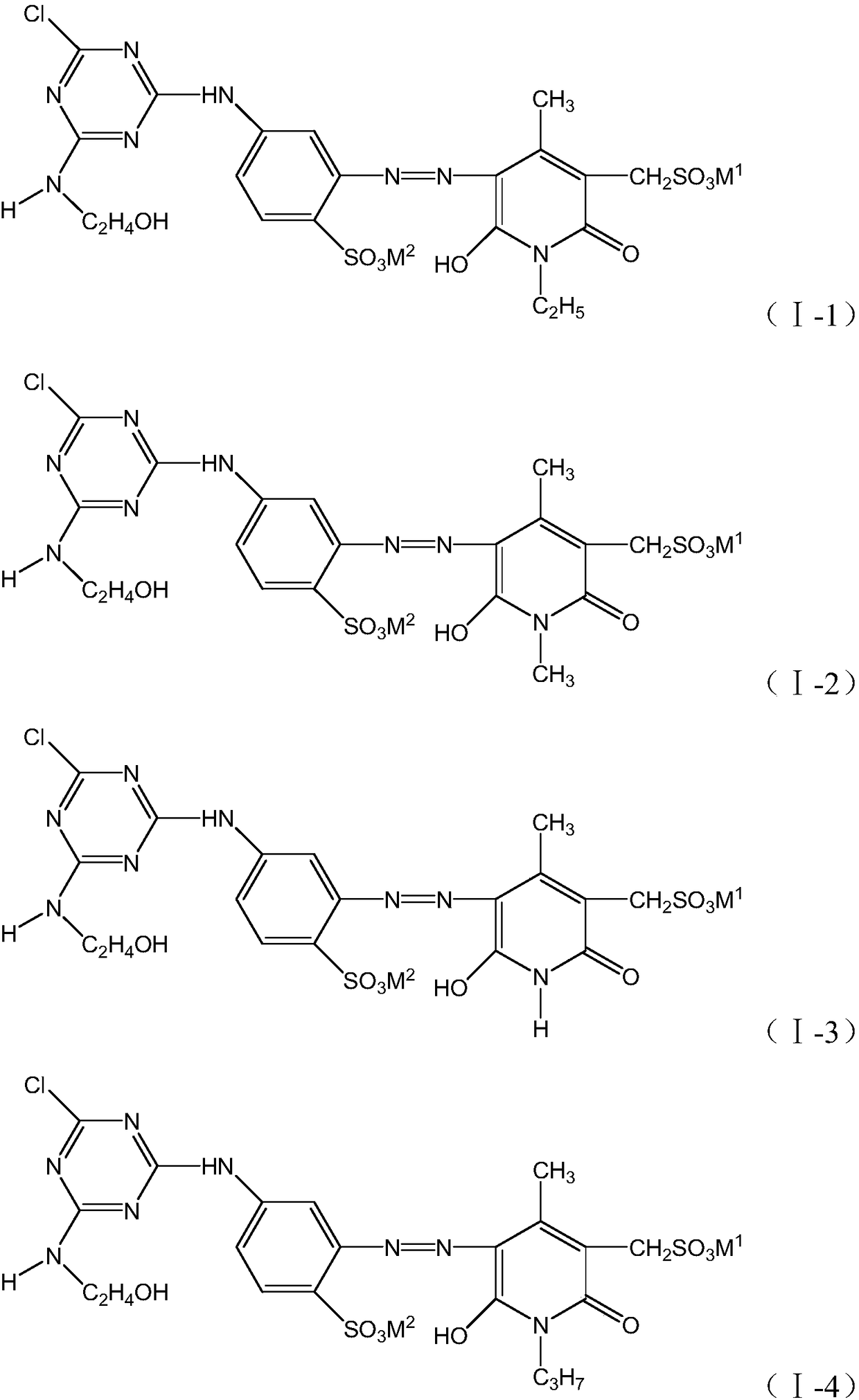
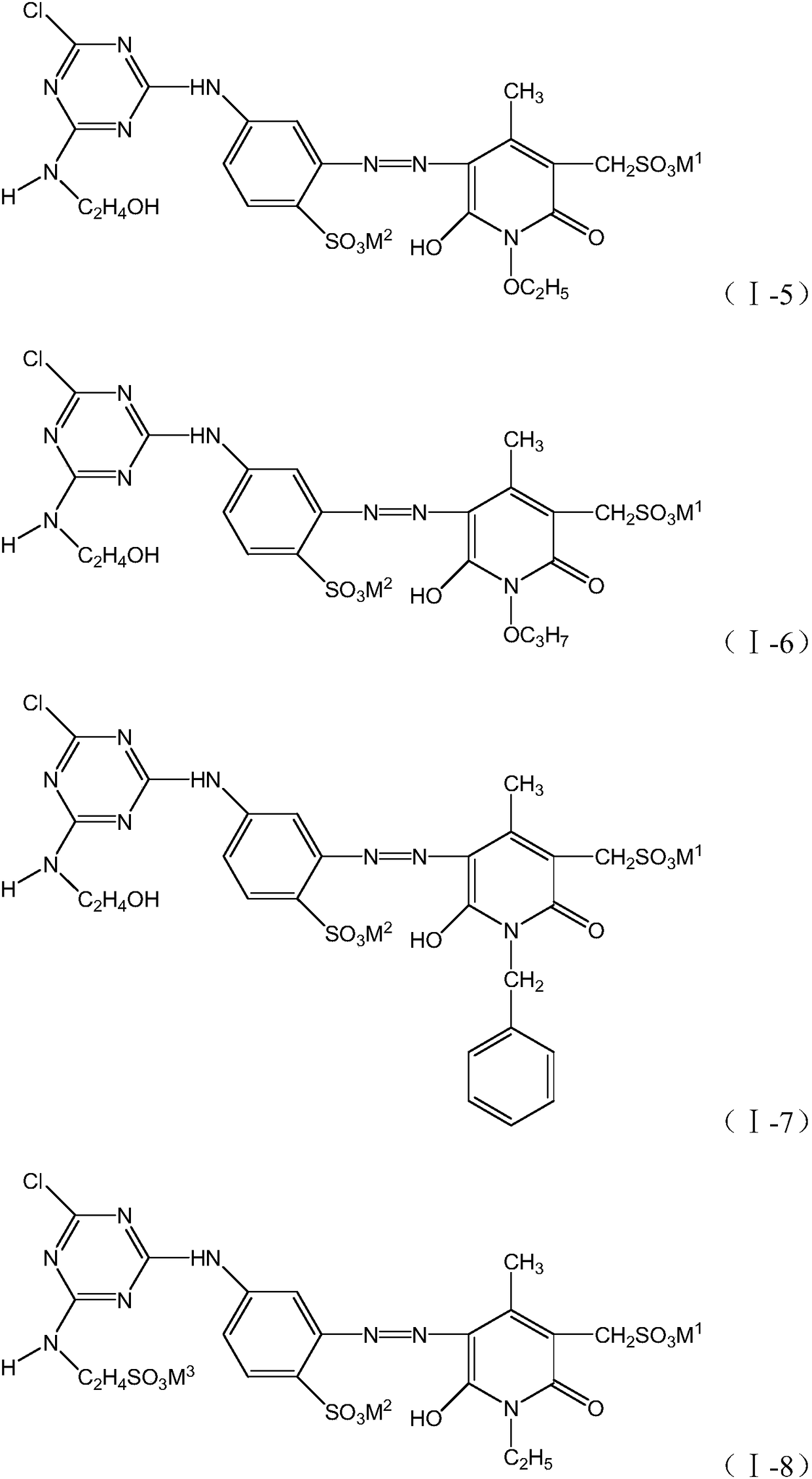
[0043] According to the preparation method in Example 1, different compounds of formula (II), formula (III) and formula (IV) are used to prepare, respectively, the structures shown in the following table 1 can be obtained:
[0044] Table 1
[0045]
[0046]
[0047]
[0048]
[0049]
[0050] Example of printing:
[0051] With the help of rapid stirring, 4 grams of the reactive dye compound dry product obtained according to Examples 1 to 15 were added to 100 grams (containing 50 grams of 4% sodium alginate thickener, 36.5 grams of water, 10 grams of urea, 1 gram of sodium alginate, etc. - sodium nitrobenzene sulfonate and 2.5 grams of sodium bicarbonate) in the original slurry, the printing paste prepared in this way is printed and dried on white cotton fabrics, and then steamed with 102 to 105 DEG C of saturated steam for 3 to 10 minutes, rinse and dry at the end.
[0052] According to GB / T3921-2008 and GB / T3920-2008, the soaping fastness and rubbing fastness...
Embodiment 1、 Embodiment 13
[0062] Embodiment 1, embodiment 13, alkali resistance stability > 120min;
[0063] Comparative example 1-3 Alkali resistance stability <120min.
[0064] (3) Lifting power: dyeing depth - K / S value
[0065] Example
[0066] In summary, the dyes of the present invention are obviously better than those of the comparative examples. For the industry, they have significantly improved color fastness (especially color fastness to rubbing), alkali resistance and lifting power.
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap