Pharmaceutical composition and preparation method for auxiliary conditioning of tumor radiotherapy and chemotherapy patients
A technology of composition and medicine, which is applied in the field of composition for auxiliary conditioning of tumor radiotherapy and chemotherapy patients, and can solve problems such as ineffective efficacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0069] The preparation technology of embodiment 1 granule of the present invention
[0070] 1. Study on the extraction and inclusion process of cinnamon
[0071] 1.1 Study on extraction process of cinnamon volatile oil
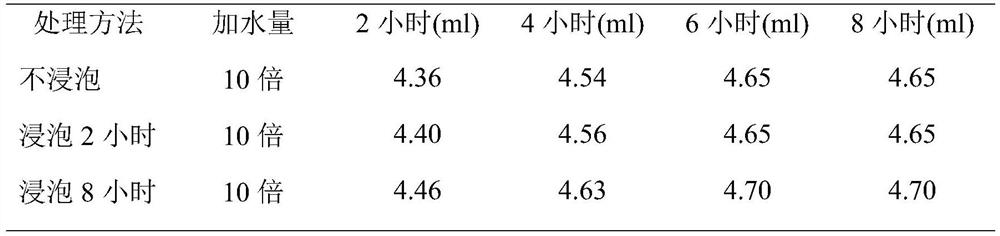
[0072] Crush the cinnamon to about 10 meshes for later use. Weigh 300 g of cinnamon coarse powder as a portion, take 3 portions in total, and investigate whether the medicinal material is soaked and the oil extraction time (steam distillation extraction). The oil output was recorded at different times until the oil output basically stopped increasing. The results are shown in Table 1.
[0073] Table 1 volatile oil extraction investigation
[0074]
[0075] From the above results, it can be seen that soaking has little effect on the extraction effect of volatile oil. The volatile oil has been basically extracted after 4 hours of extraction from medicinal materials, and there is only a small increase in 6 hours. After 8 hours, the amount of oil has no long...
Embodiment 2
[0196] The stability experiment of embodiment 2 granules of the present invention
[0197] The granules of the pharmaceutical composition of the present invention are tested for the content of crude polysaccharides, adenosine, and astragaloside IV at different times under airtight, dark, and normal temperature conditions. The detection items, basis, and results are as follows:
[0198] Inspection items and basis: crude polysaccharide - Q / QHCT004-2015 (enterprise standard)
[0199] Adenosine—health food inspection and technical evaluation specification (2003)
[0200] Astragaloside IV——Q / QHCT004-2015 (enterprise standard)
[0201] Sample placement conditions: constant temperature 38°C, relative humidity 75%.
[0202] Month Zero Results
[0203]
[0204] first month results
[0205]
[0206] second month results
[0207]
[0208] 3rd month results
[0209]
Embodiment 3
[0210] Example 3 Verification of the daily dosage of the pharmaceutical composition of the present invention
[0211] The daily dosage of the pharmaceutical composition of the present invention and the reference daily dosage of the Pharmacopoeia are shown in Table 22:
[0212] Table 22 Daily dosage of the present invention and Pharmacopoeia reference daily dosage
[0213] raw material Daily dosage of the present invention Reference daily dosage of medicinal materials Cordyceps sinensis 0.5g 3~9g Broken Ganoderma Lucidum Spore Powder 1.5g 6~12g angelica 1g 6~12g Astragalus 5g 9~30g Cinnamon 1g 1~5g licorice 3g 2~10g
[0214] It can be seen from the above that the pharmaceutical composition of the present invention adopting the above formula, Cordyceps sinensis, Ganoderma lucidum spore powder, Angelica sinensis and Radix Astragali are significantly lower than the pharmacopoeia reference daily dose. Therefore, in order ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com