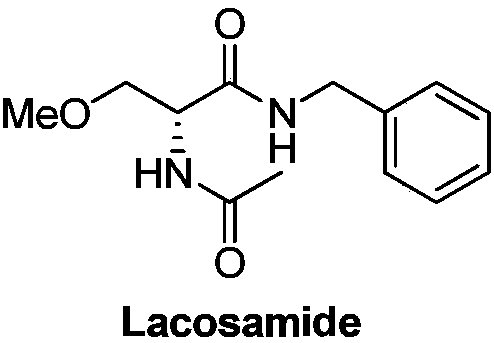
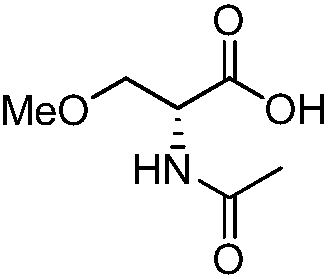
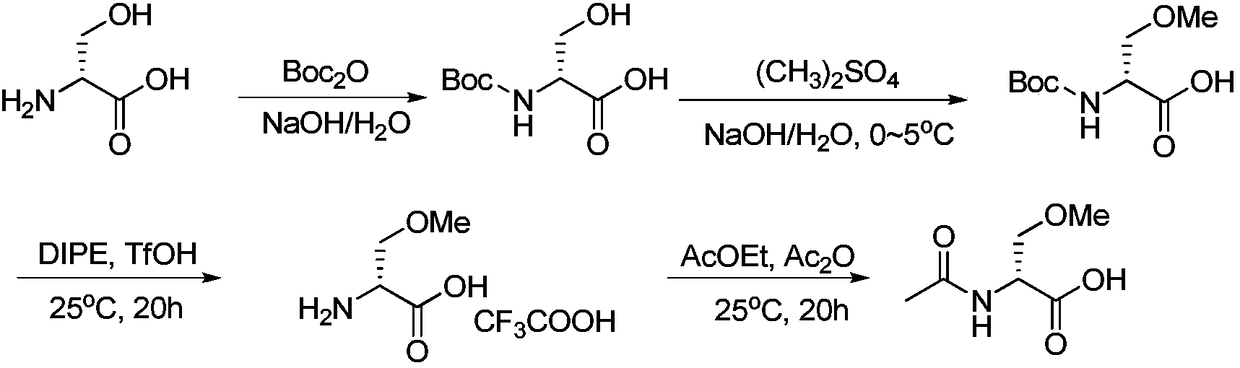
Preparation method of R-2-acylamino-3-methyl methoxypropionate
A technology of methyl methoxypropionate and acylamino, which is applied in the field of preparation of lacosamide intermediates, achieving the effects of mild reaction conditions, high yield and simple post-treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] (1) According to the amount ratio of N-acetylglycine: bis(trichloromethyl)carbonate: N,N-dimethylformamide is 1.0:0.32:1.0; in a 1L dry reaction bottle, add N - Acetylglycine 35.1g, N,N-dimethylformamide 21.9g, control the reaction temperature at 0-5°C; dissolve 28.5g of bis(trichloromethyl)carbonate in 285mL of n-hexane, add dropwise to the reaction bottle; after the dropwise addition, raise the temperature and react at 60°C for 12 hours. After the reaction, add the reaction solution into the ice-water mixture, adjust the pH to 8 with ammonia water, separate layers, take the organic phase, and evaporate to dryness under reduced pressure 27.9 g of white solid (E)-4-((dimethylamino)methylene)-2-methyloxazol-5(4H)-one was obtained, with a yield of 60%;
[0045] Step (2): In a 1L dry reaction flask, add the intermediate (E)-4-((dimethylamino)methylene)-2-methyloxazole-5( 4H)-ketone 23.1g and methanol 70mL, add 2N NaOH aqueous solution 75mL at room temperature; stir overni...
Embodiment 2
[0049] (1) According to the amount ratio of N-acetylglycine: bis(trichloromethyl)carbonate: N,N-dimethylformamide is 1.0:0.5:1.5 feeding; in a 1L dry reaction bottle, add N - Acetylglycine 35.1g, N,N-dimethylformamide 32.9g, control the reaction temperature at 0-5°C; dissolve 44.5g of bis(trichloromethyl)carbonate in 350mL of cyclohexane, add dropwise Reaction bottle; after the dropwise addition, raise the temperature and react at 20°C for 24 hours. After the reaction, add the reaction liquid to the ice-water mixture, adjust the pH to 8 with ammonia water, separate layers, take the organic phase, and evaporate under reduced pressure. Dry to obtain 24.6 g of (E)-4-((dimethylamino)methylene)-2-methyloxazol-5(4H)-one as a white solid, with a yield of 53%;
[0050] Step (2): In a 1L dry reaction flask, add the intermediate (E)-4-((dimethylamino)methylene)-2-methyloxazole-5( 4H)-ketone 23.1g and ethanol 140mL, add 2N NaOH aqueous solution 225mL at room temperature; stir overnight,...
Embodiment 3
[0054] (1) According to the amount ratio of N-acetylglycine: bis(trichloromethyl)carbonate N,N-diethylformamide is 1.0:0.7:3.0; in a 1L dry reaction bottle, add N- Acetylglycine 35.1g, N,N-diethylformamide 91.0g, control the reaction temperature at 0-5°C; dissolve 62.3g of bis(trichloromethyl)carbonate in 300mL of dichloromethane, add dropwise to the reaction bottle; after the dropwise addition, raise the temperature and react at 40°C for 16 hours. After the reaction, add the reaction solution into the ice-water mixture, adjust the pH to 8 with ammonia water, separate layers, take the organic phase, and evaporate to dryness under reduced pressure Obtained 44.6 g of white solid (E)-4-((diethylamino)methylene)-2-methyloxazol-5(4H)-one, yield 82%;
[0055] Step (2): In a 1L dry reaction flask, add the intermediate (E)-4-((diethylamino)methylene)-2-methyloxazole-5( 27.3g of 4H)-ketone and 270mL of acetonitrile, 225mL of 2N KOH aqueous solution was added at room temperature; stirr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com