Nintedanib inclusion compound and preparation and preparation method thereofof preparation
A technology of nintedanib and clathrates, which is applied in the directions of pharmaceutical formulation, pill delivery, drug combination, etc., can solve the problems of residual organic solvents, high equipment cost, and difficulty in scale-up production, so as to promote drug efficacy, improve solubility, Ease of production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
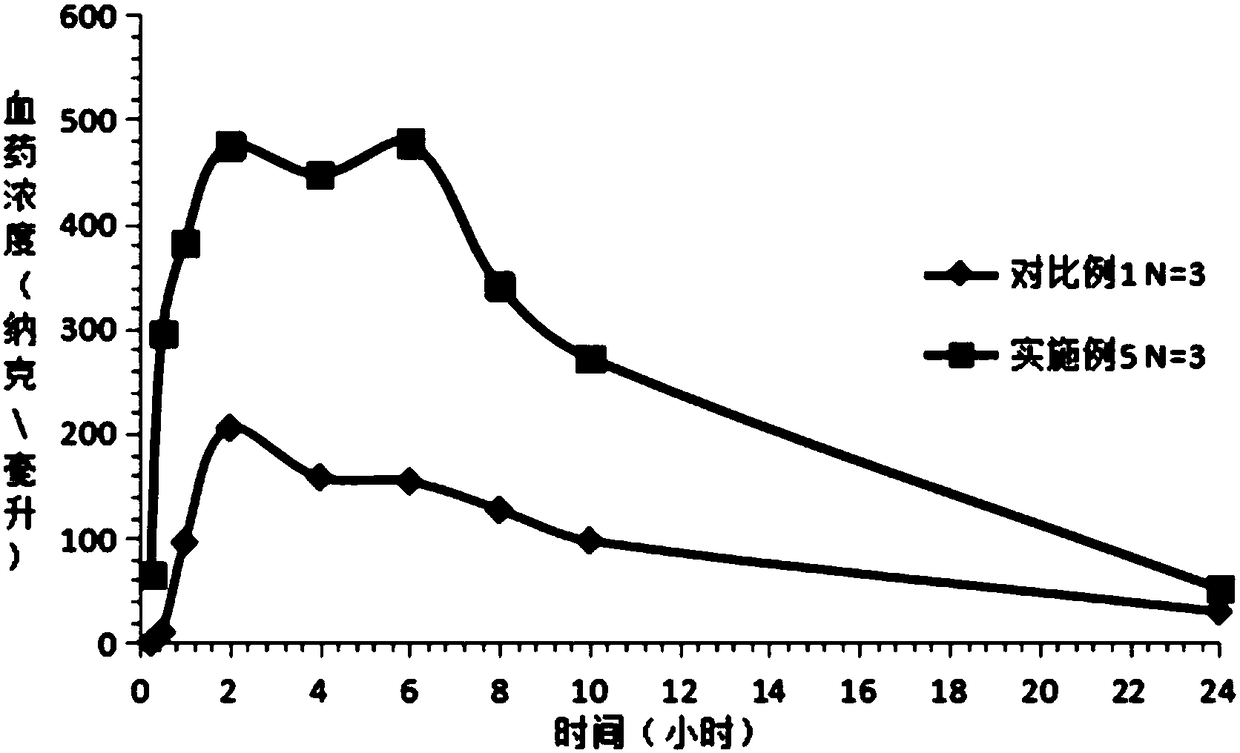
Image
Examples
Embodiment 1
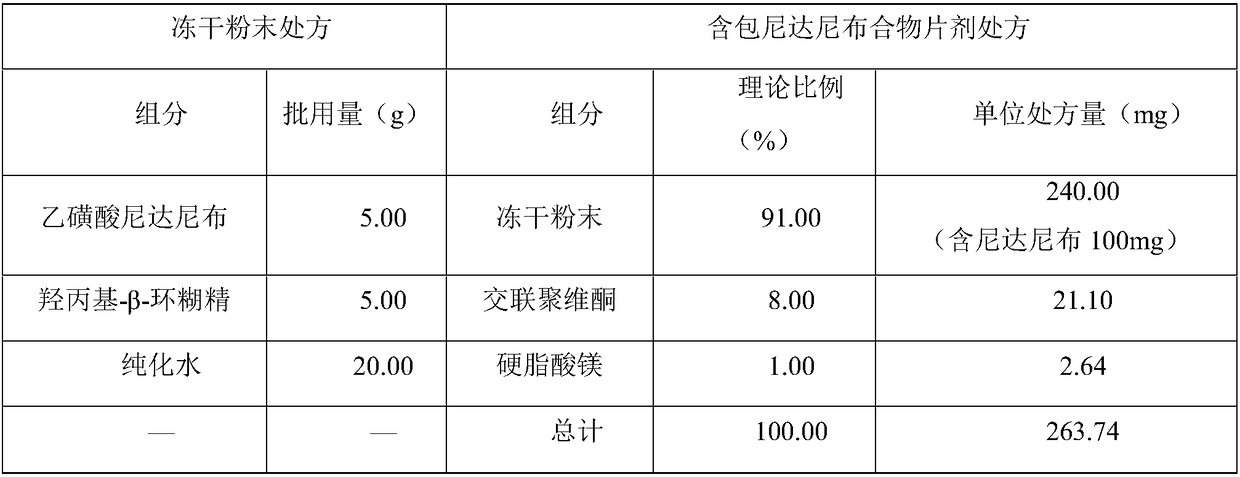
[0056] In this example, the purified water and hydroxypropyl-β-cyclodextrin were weighed according to the prescription ratio in Table 4 to make them fully dissolved, then slowly added nintedanib ethanesulfonate under stirring in a water bath at 55°C, and continued After stirring for 4 hours, it was divided into vials and freeze-dried. The freeze-dried samples were transferred from the vials and ground in a mortar to obtain a freeze-dried powder. The ingredients were mixed according to the prescription ratio in Table 4 for 5 minutes, and tableted to prepare nintedanib inclusion compound tablets.
[0057] The prescription form of table 4 embodiment 1
[0058]
Embodiment 2
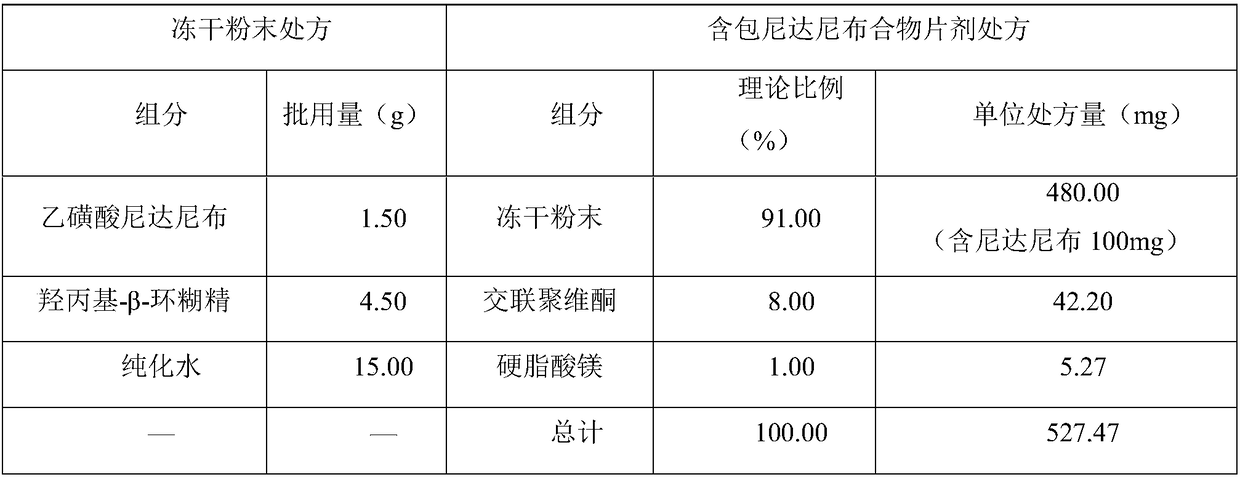
[0060] In this example, the purified water and sulfobutyl ether-β-cyclodextrin were weighed according to the prescription ratio in Table 5 to make them fully dissolved, then slowly added nintedanib ethanesulfonate under stirring at room temperature, and continued to stir for 6 hours Finally, it is divided into vials and freeze-dried. The freeze-dried samples are transferred from the vials and ground in a mortar to obtain a freeze-dried powder. The ingredients were mixed according to the prescription ratio in Table 5 for 5 minutes, and tableted to prepare nintedanib inclusion compound tablets.
[0061] The prescription form of table 5 embodiment 2
[0062]
[0063]
Embodiment 3
[0065] In this example, weigh the purified water and sulfobutyl ether-β-cyclodextrin according to the prescription ratio in Table 6 to make them fully dissolved, slowly add nintedanib ethanesulfonate under stirring at room temperature, and continue stirring for 6 hours Finally, the inclusion solution of nintedanib ethanesulfonate was obtained. The nintedanib ethanesulfonate inclusion solution is used as the fluidized bed granulation liquid, and the microcrystalline cellulose is used as the granulation substrate for fluidized bed granulation to obtain the nintedanib ethanesulfonate dry granules, and then add the prescription amount The crospovidone and magnesium stearate are compressed into tablets to prepare nintedanib inclusion compound tablets.
[0066] The prescription form of table 6 embodiment 3
[0067] components
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com