Compound dimethoxystyryl-amino-benzimidazolyl-triazine and salt thereof as well as preparation method and application
A technology based on dimethoxystyrene and benzimidazole, which is applied in the field of biopharmaceuticals to achieve the effects of low equipment requirements, large-scale production, and simple processes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Compound 2-[(E)-3',4'-dimethoxystyryl]-4-amino-[1.2]-benzoimidazolyl-[1,3,5]triazine (referred to as G03) preparation of
[0045] The chemical synthesis route is as follows:
[0046]
[0047] Preparation of 2-[(E)-3',4'-dimethoxystyryl]-4-amino-[1.2]-benzimidazolyl-[1,3,5]triazine, specific synthesis steps as follows:
[0048] (1) Synthesis of 2-methyl-1,3-benzoxazol-4-one perchlorate
[0049] Add 6.85g of salicylamide and 30mL of acetic anhydride in a 100mL four-neck flask, stir for 10min, then add 5mL of perchloric acid (70wt%), react at room temperature, and precipitate a solid quickly, add diethyl ether to mix and filter, and wash the solid with diethyl ether. Dry to obtain off-white product 12.0g, yield 92%, 205 ℃ of melting points (Ryabukhin, Yu.I., Mezheritskii, V.V., Karpenko, V.D., and Dorofeenko, G.N., Khim.Geterotsikl.Soedin., 1975, p.1184. );
[0050] (2) Synthesis of (E)-2-(3',4'-dimethoxystyryl)-1,3-benzoxazol-4-one perchlorate
[0051] Add 2-meth...
Embodiment 2
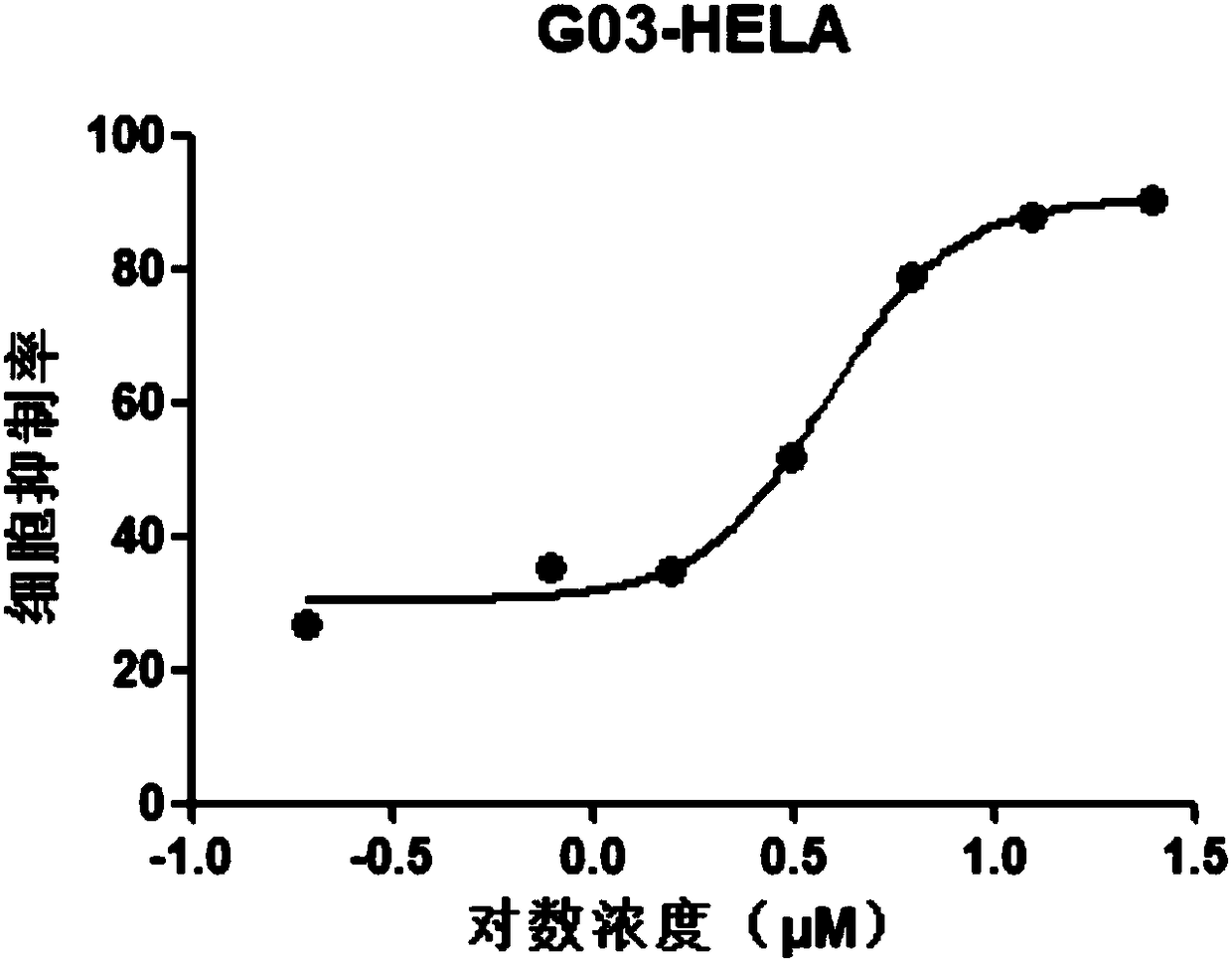
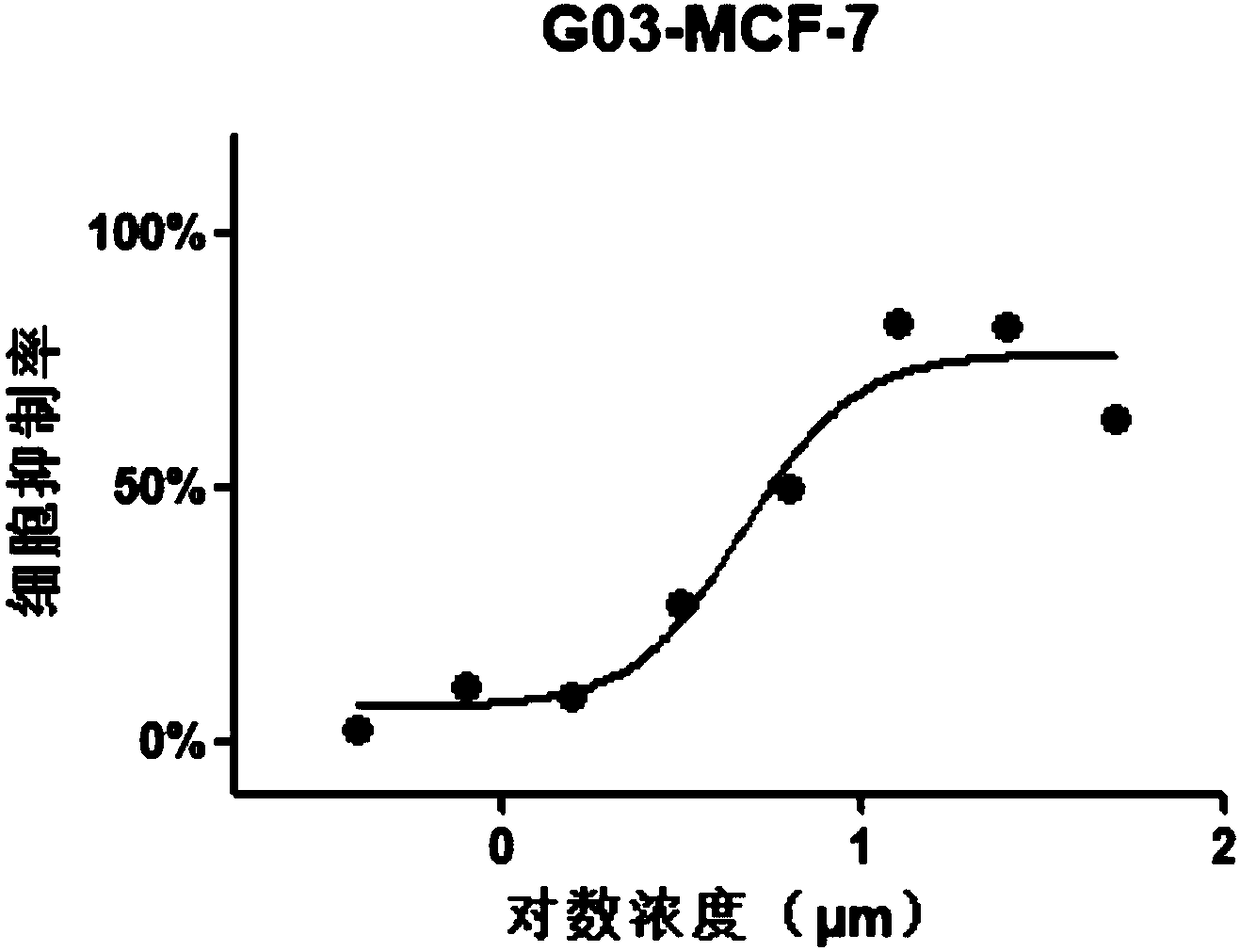
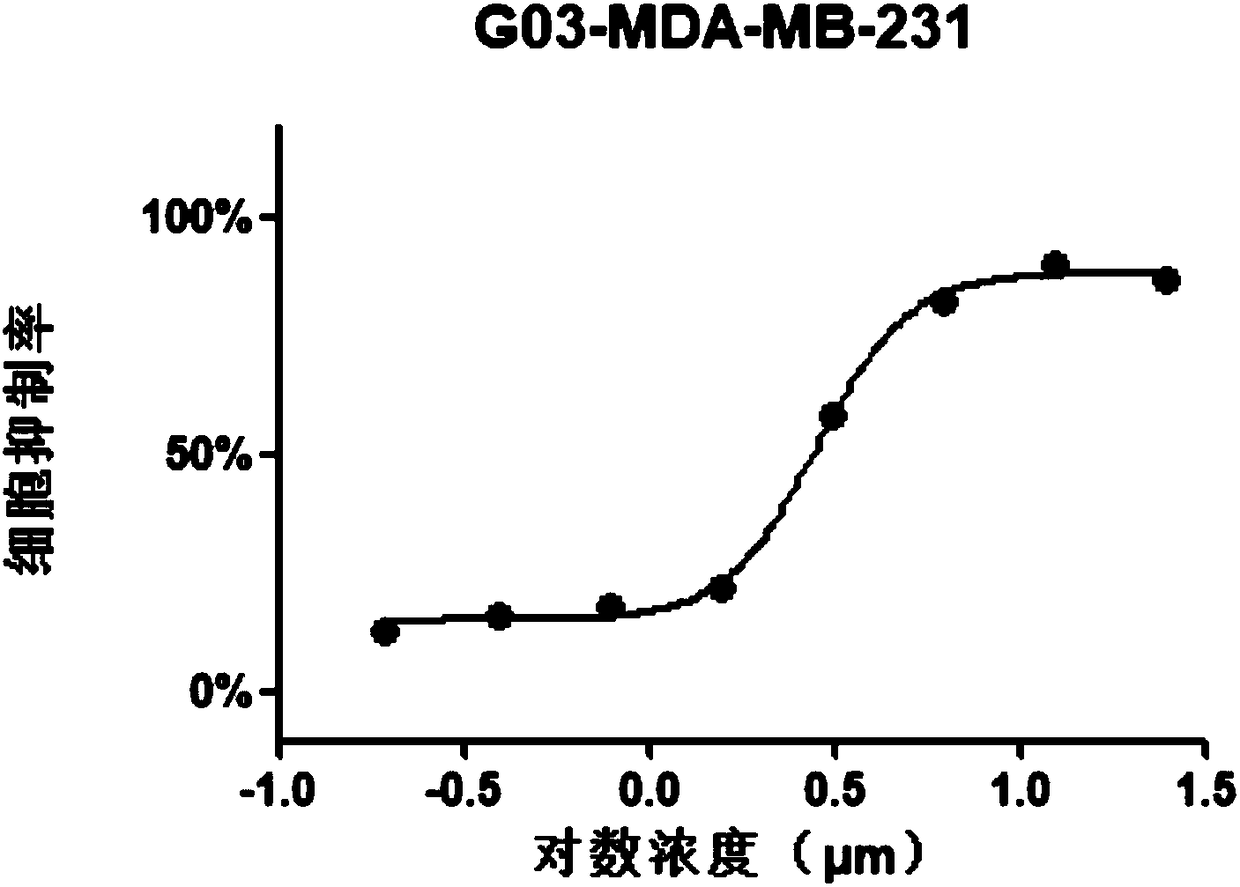
[0056] MTT (3 -(4,5-dimethylthiazole-2)-2,5-diphenyltetrazolium bromide) experiment
[0057] (1) Plating: Hela, MCF-7, MDA-MB-231, HCT116 and HepG2 cell lines in logarithmic growth phase were taken, and 3500 cells / well were spread in 96-well plates, 100 μl per well;
[0058] (2) Dosing: After 24 hours of adherence, the original culture medium was carefully sucked away, and the medicine was added, 100 μl per well; a medicine-dosing group, a control group, and a blank group were set;
[0059] Dilute the compound 2-[(E)-3',4'-dimethoxystyryl]-4-amino-[1.2]-benzimidazolyl-[1,3,5]triazine with medium Dosing at 100uM, 50uM, 25uM, 12.5uM, 6.25uM, 3.125uM, 1.5625uM and 0.78125uM, with the highest concentration not exceeding 100uM; the control group was added with blank medium; the blank group was the control group without cells;
[0060] (3) Incubation: at 5vol% CO 2 , Incubate at 37°C for 48h;
[0061] (4) Add MTT: add MTT (2.5 mg / ml) at 20 μl per well, and place in an incubator ...
Embodiment 3
[0069] Immunofluorescence experiment
[0070] (1) Plating: take the Hela cell line cells in the logarithmic growth phase, and plate at a density of 50vol% at the bottom of each well in a 24-well plate;
[0071] (2) Dosing: After the cells adhere to the wall overnight, dosing according to the concentration gradient of 2.5uM, 5uM and 10uM, the solvent control group was given blank medium, and the positive control group was given 10uM paclitaxel;
[0072] (3) Incubation: at 5vol% CO 2 , Incubate at 37°C for 4h, 8h, and 24h time points.
[0073] (4) After the incubation is completed, the culture medium is discarded, and washed twice with PBS, each time for 5 minutes;
[0074] (5) Fixation and permeabilization: 400 μl ice methanol per well, permeabilization for 5 minutes;
[0075] (6) Blocking: 2.5 wt% BSA was prepared in PBS, centrifuged at 13000 rpm for 10 min before use, and 300 μl BSA per well was blocked at room temperature for 25 min;
[0076] (7) Incubate the primary ant...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com