A new application of pyridine compounds
A compound and pyridine technology, applied in the field of pyridine compounds, can solve the problems of poor patient compliance, large toxic and side effects, and high infection risk, and achieve the effects of low dosage, low toxic and side effects, and convenient use.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] This example studies the effect of pyrazolopyridine compounds and imidazopyridine compounds of the present invention on inhibiting Mycobacterium bruli's necrosis in vitro.
[0040] (1) Experimental grouping
[0041] Solvent group: give an equal volume of drug solvent as a control;
[0042] Pyrazolopyridine compound group: With the skeleton shown in formula (I), the structures of R1, R2 and R3 of each compound are shown in Table 1:
[0043] Table 1 Structural formula of pyrazolopyridine compounds
[0044] Compound number R1 R2 R3 1 5-Me Me CF 3
2 5-Me Me Formula (a) 3 5-Me Me Formula (b) 4 5-Me Me Formula (c) 5 5-Me Me Formula (d) 6 5-Me Me Formula (e) 7 5-Me Me Formula (f) 8 4-Me Me Formula (f) 9 6-Me Me Formula (f) 10 7-Me Me Formula (f) 11 5-OMe Me Formula (f) 12 5-Cl Me Formula (f) 13 5-Et Me Formula (f) 14 5-i-Pr ...
Embodiment 2
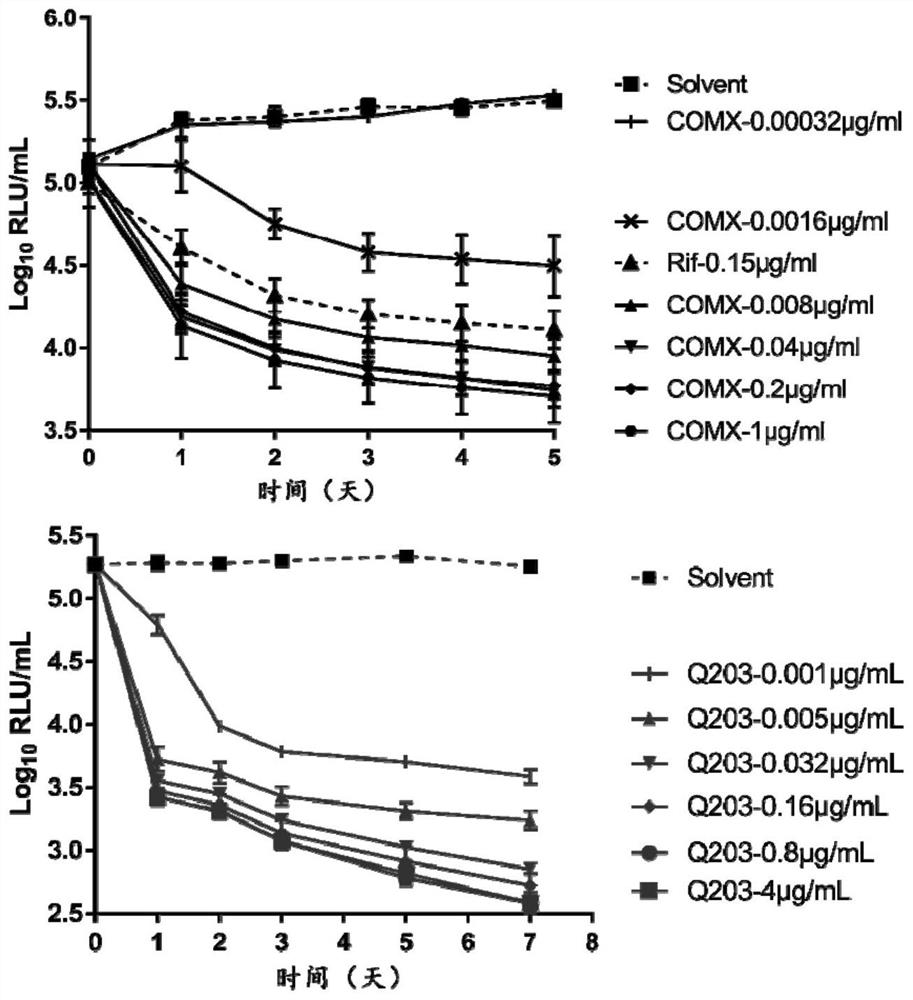
[0064] This example studies the effect of COMX and Q203 on inhibiting Mycobacterium brulii necrosis in vitro.
[0065] (1) Experimental grouping
[0066] Solvent group: give an equal volume of drug solvent as a control;
[0067] COMX group: concentrations of 1 μg / mL, 0.2 μg / mL, 0.04 μg / mL, 0.008 μg / mL, 0.0016 μg / mL, 0.00032 μg / mL;
[0068] Group Q203: concentration of 4μg / mL, 0.8μg / mL, 0.16μg / mL, 0.03μg / mL, 0.005μg / mL; 0.001μg / mL
[0069] The COMX and Q203 used above were dissolved in DMSO for use.
[0070] (2) Experimental steps
[0071] 1. For the cultivation of self-luminescent Mycobacterium bruley necrosis, the experimental steps are the same as in Example 1.
[0072] 2. Adding drugs and co-incubating for continuous detection
[0073] Add 198 μL of bacterial solution and 2 μL of the prepared drug to each EP tube, and at the same time, use a pipette to mix the drug and the bacterial solution evenly. Test the bactericidal or antibacterial effect of the drug: put the EP...
Embodiment 3
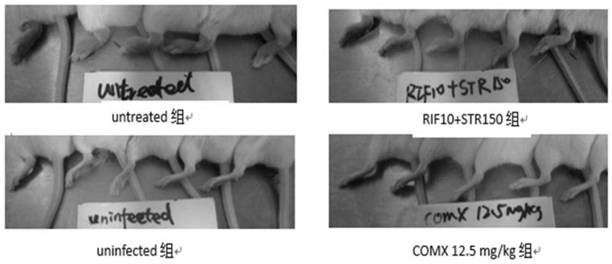
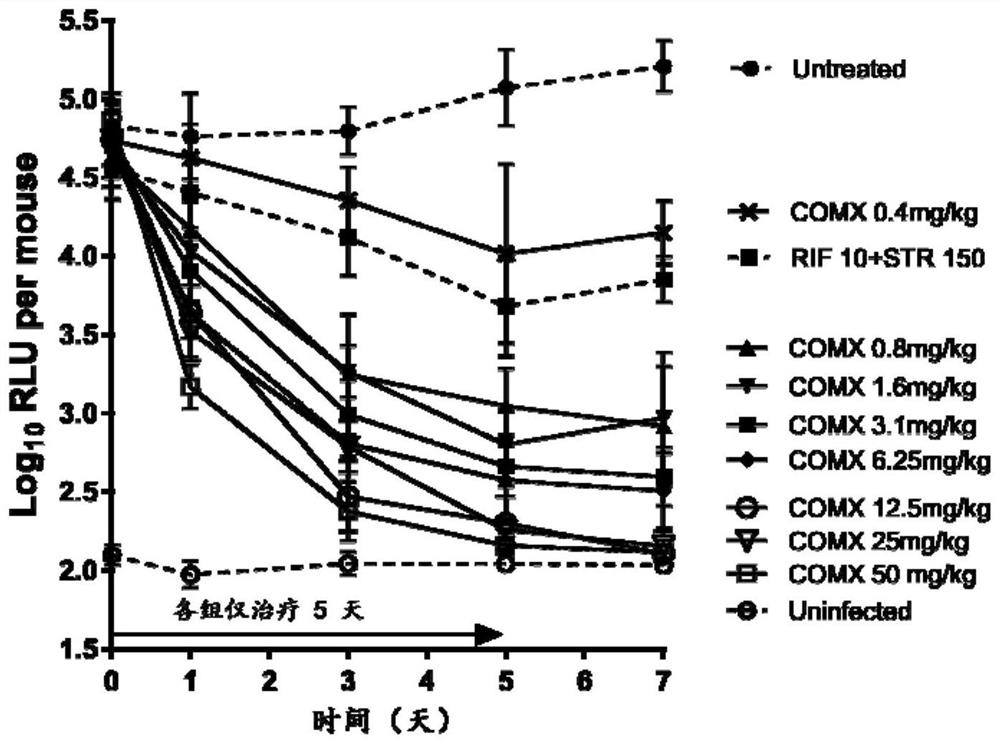
[0078] This example studies the inhibitory effect of COMX and Q203 on Mycobacterium brulii necrosis in mice.
[0079] (1) Grouping and administration of animals
[0080] Select Balb / C mice about 6 weeks old, inject the mice with self-luminous Mycobacterium brullii necrosis infection into the hind feet, and start the drug treatment 10 days after the infection. The drug groups are as follows, with 10 mice in each group:
[0081] Untreated group: given an equal volume of deionized water after infection as a negative control;
[0082] uninfected group: uninfected bacteria group, as the background baseline of the detected luminescence;
[0083] Rifampicin + streptomycin group: Rifampicin 10mg / kg by intragastric administration and streptomycin 150mg / kg by subcutaneous injection;
[0084] COMX group: COMX oral administration doses were 50mg / kg, 25mg / kg, 12.5mg / kg, 6.25mg / kg, 3.2mg / kg, 1.6mg / kg, 0.8mg / kg, 0.4mg / kg;
[0085] Q203 group: Q203 intragastric administration doses were 25...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com