Drug sustained-release material and preparation method thereof
A sustained-release material and drug technology, applied in the direction of non-active ingredients of polymer compounds, inorganic non-active ingredients, organic non-active ingredients, etc., can solve problems such as reduction, increase of drug level, and insufficient therapeutic effect, and achieve hydrophilicity Good performance, high encapsulation rate, and good drug sustained release effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
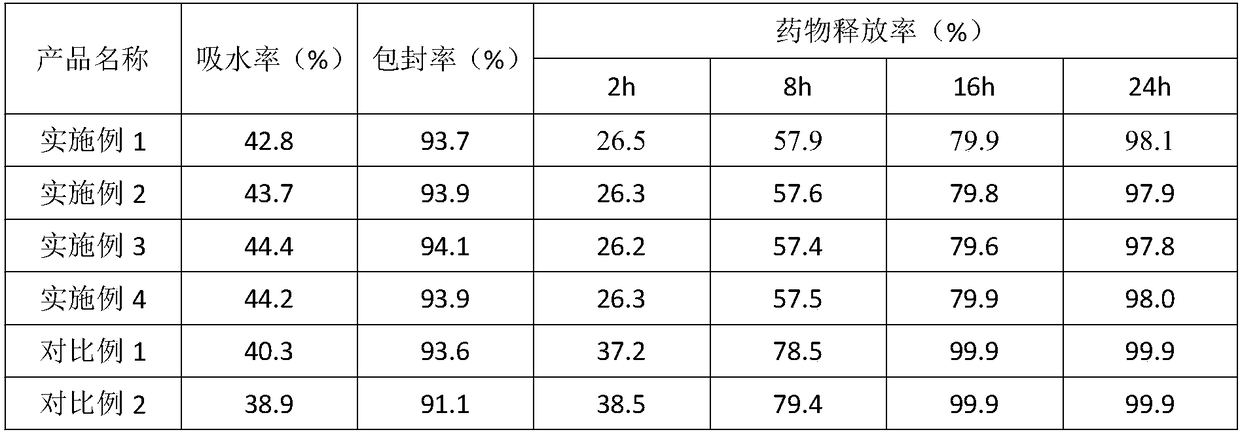
[0018] A drug slow-release material, prepared from the following ingredients in parts by weight: 0.5 parts of silk fibroin peptide, 1 part of polycaprolactone, 1 part of bovine serum albumin, 1 part of glycolic acid, 2 parts of garlic oil, sodium tripolyphosphate 0.5 parts, 1 part of aloe polysaccharide, 3 parts of low molecular weight chitosan, 2 parts of polyanionic cellulose, 1 part of N,N'-dioleoyl ethylenediamine diethanesulfonate, 5 parts of polyethylene glycol, 2 parts of dialdehyde, 50 parts of deionized water.
[0019] The preparation method of above-mentioned drug sustained-release material is:
[0020] (1) Mix and stir low molecular weight chitosan, polyanionic cellulose, silk fibroin peptide, polycaprolactone, bovine serum albumin, glycolic acid, aloe polysaccharide and deionized water for 20 minutes to obtain component A;
[0021] (2) Mix and stir garlic oil, N,N'-dioleoyl ethylenediamine diethanesulfonate sodium and polyethylene glycol for 10 minutes to obtain c...
Embodiment 2
[0027] A drug slow-release material, prepared from the following ingredients in parts by weight: 0.8 parts of silk fibroin peptide, 1.5 parts of polycaprolactone, 1.5 parts of bovine serum albumin, 1.5 parts of glycolic acid, 2.5 parts of garlic oil, sodium tripolyphosphate 0.7 parts, 1.5 parts of aloe polysaccharide, 4 parts of low molecular weight chitosan, 2.5 parts of polyanionic cellulose, 2 parts of N,N'-dioleoyl ethylenediamine diethanesulfonate, 6 parts of polyethylene glycol, 2.5 parts of dialdehyde, 55 parts of deionized water.
[0028] The preparation method of above-mentioned drug sustained-release material is:
[0029] (1) Mix and stir low molecular weight chitosan, polyanionic cellulose, silk fibroin peptide, polycaprolactone, bovine serum albumin, glycolic acid, aloe polysaccharide and deionized water for 25 minutes to obtain component A;
[0030] (2) Mix garlic oil, sodium N,N'-dioleoyl ethylenediamine diethanesulfonate and polyethylene glycol for 15 minutes t...
Embodiment 3
[0036] A drug slow-release material, prepared from the following ingredients in parts by weight: 1 part of silk fibroin peptide, 1.5 parts of polycaprolactone, 2 parts of bovine serum albumin, 1.5 parts of glycolic acid, 3 parts of garlic oil, sodium tripolyphosphate 0.8 parts, 1.5 parts of aloe polysaccharide, 4.5 parts of low molecular weight chitosan, 2.5 parts of polyanionic cellulose, 2 parts of N,N'-dioleoyl ethylenediamine diethanesulfonate, 6 parts of polyethylene glycol, 3 parts of dialdehyde, 60 parts of deionized water.
[0037] The preparation method of above-mentioned drug sustained-release material is:
[0038] (1) Mix and stir low molecular weight chitosan, polyanionic cellulose, silk fibroin peptide, polycaprolactone, bovine serum albumin, glycolic acid, aloe polysaccharide and deionized water for 25 minutes to obtain component A;
[0039] (2) Mix garlic oil, sodium N,N'-dioleoyl ethylenediamine diethanesulfonate and polyethylene glycol for 15 minutes to obtai...
PUM
| Property | Measurement | Unit |
|---|---|---|
| water absorption | aaaaa | aaaaa |
| encapsulation rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com