Method for synthesizing ternary precursor without inert gas protection
An inert gas, precursor technology
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
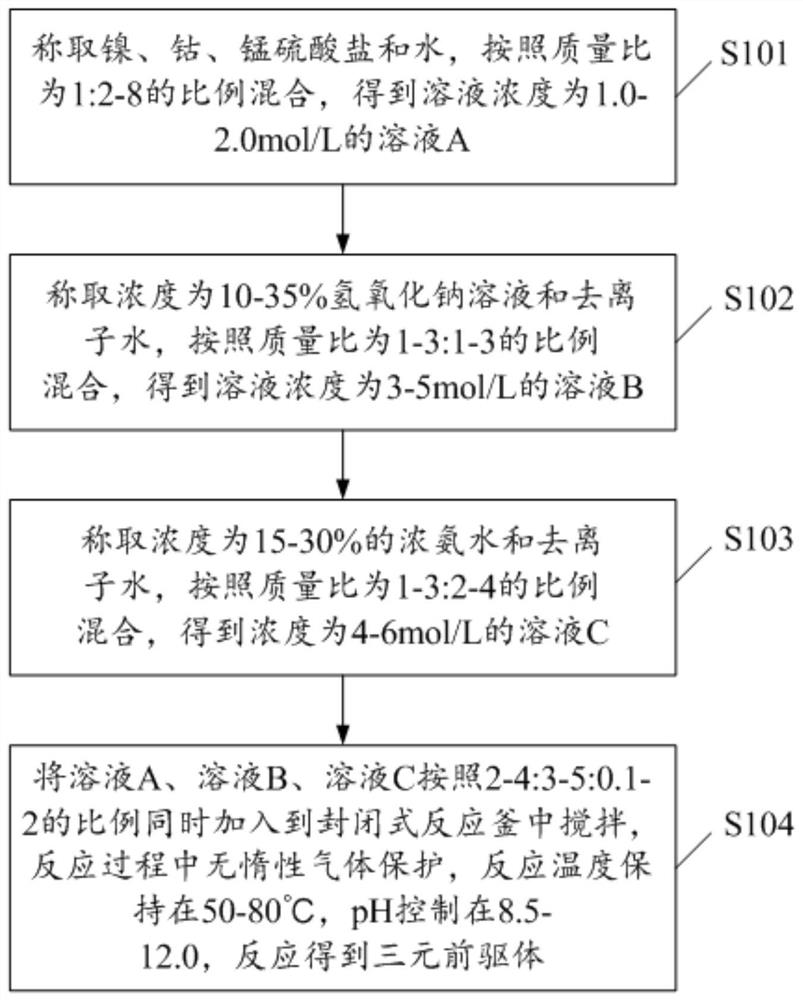
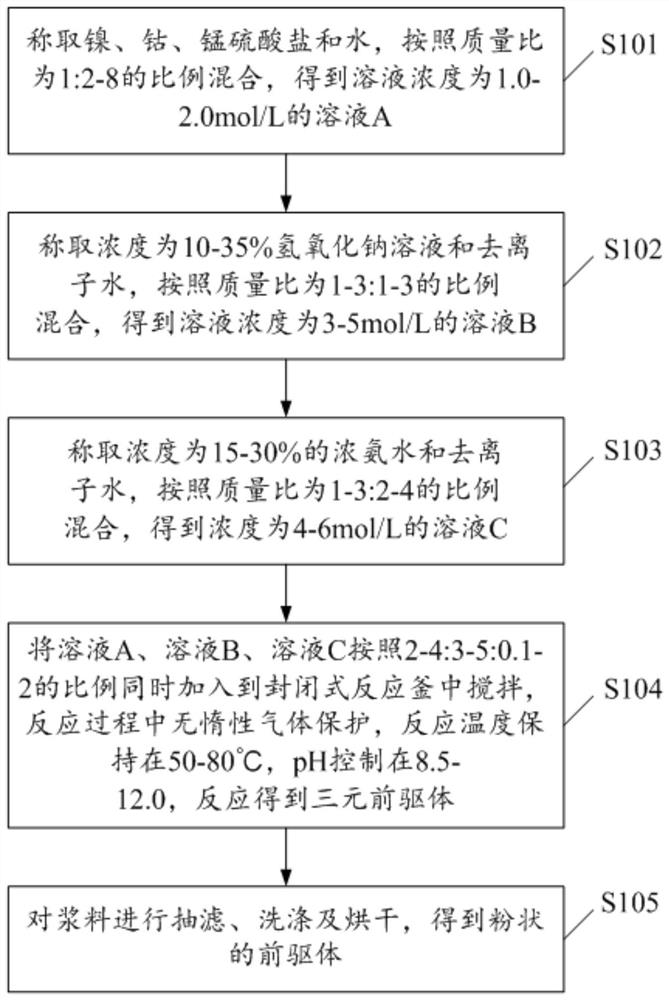
[0054] 1. Weigh 300g of nickel sulfate, 200g of cobalt sulfate, and 50g of manganese sulfate and dissolve them in 1100g of water. After stirring evenly, a solution A with a solution concentration of 1.98mol / L is obtained.
[0055] 2. Weigh 200 g of 15% sodium hydroxide solution and 200 g of deionized water, and stir evenly to obtain solution B with a solution concentration of 3.75 mol / L.
[0056] 3. Weigh 120 g of concentrated ammonia water with a concentration of 25% and 200 g of deionized water to obtain solution C with a solution concentration of 4.28 mol / L.
[0057] 4. Weigh 300g, 400g, and 100g of the above solution A, solution B, and solution C respectively.
[0058] 5. Put solution A, solution B, and solution C into the closed reactor in parallel, and the reaction process does not need nitrogen protection. The stirring rate of the reactor was controlled at 600 rpm / min, the reaction temperature was kept at 50° C., the pH was kept at 9-10, and the reaction time was 40 h....
Embodiment 2
[0061] 1. Weigh 350g of nickel sulfate, 200g of cobalt sulfate, and 60g of manganese sulfate and dissolve them in 2000g of water. After stirring evenly, a solution A with a solution concentration of 1.22mol / L is obtained.
[0062] 2. Weigh 150 g and 200 g of 20% sodium hydroxide solution and 200 g of deionized water, and stir evenly to obtain solution B with a solution concentration of 3.75 mol / L.
[0063] 3. Weigh 120 g of concentrated ammonia water with a concentration of 24% and 200 g of deionized water to obtain solution C with a solution concentration of 4.11 mol / L.
[0064] 4. Weigh 300g, 450g and 100g of the above solution A, solution B and solution C respectively.
[0065] 5. Put solution A, solution B, and solution C into the closed reactor in parallel, and the reaction process does not need nitrogen protection. The stirring rate of the reactor was controlled at 700 rpm / min, the reaction temperature was kept at 60° C., the pH was kept at 9-11, and the reaction time w...
Embodiment 3
[0068] 1. Weigh 320g of nickel sulfate, 250g of cobalt sulfate, and 80g of manganese sulfate and dissolve them in 2000g of water. After stirring evenly, a solution A with a solution concentration of 1.32mol / L is obtained.
[0069] 2. Weigh 200 g of 20% sodium hydroxide solution and 200 g of deionized water, and stir evenly to obtain solution B with a concentration of 5 mol / L.
[0070] 3. Weigh 150 g of concentrated ammonia water with a concentration of 15% and 160 g of deionized water to obtain solution C with a solution concentration of 4.0 mol / L.
[0071] 4. Weigh 350g, 450g, and 150g of the above solution A, solution B, and solution C respectively.
[0072] 5. Put solution A, solution B, and solution C into the closed reactor in parallel, and the reaction process does not need nitrogen protection. The stirring rate of the reactor was controlled at 800 rpm / min, the reaction temperature was kept at 70° C., the pH was kept at 9-10, and the reaction time was 35 hours.
[0073...
PUM
| Property | Measurement | Unit |
|---|---|---|
| electrical conductivity | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com