Rabies immunogenic conjugate
A technology of immunogenicity and conjugates, applied in the medical field, can solve the problems of highly dangerous parts, inability to produce effective protection, backwardness, etc., and achieve the effect of enhancing immunogenicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0229] Rabies whole virus particle antigen stock solution preparation and vaccine preparation:
[0230] Cells from the Vero cell working bank were recovered and expanded at 37°C, and after 3-5 generations of continuous passage, the cell density reached 1.0×10 5 ~1.0×10 6 / ml, inoculated into a bioreactor containing 1-25g / L microcarriers for tank-flow culture. Microcarrier high-density suspension culture for 5 to 7 days, the cell density reaches 1.0×10 6 ~1.0×10 7 / ml, replace with maintenance solution. Inoculate the virus titer at 0.001~0.1MOI not less than 7.5lg LD 50 / ml of rabies virus fixed virus CTN‐1V strain. Replace with maintenance medium, and culture in tank flow at 33-35°C. The virus fluid can be continuously harvested on the third day, and the virus fluid can be continuously harvested for more than 20 days. The harvested virus fluid is clarified and filtered, and concentrated by 100-300KD membrane ultrafiltration for 10-30 times. Add β‐propiolactone at a rat...
Embodiment 2
[0233] Preparation of carrier protein‐tetanus toxoid (TT)
[0234] Open the tetanus freeze-dried bacterial strain preserved at low temperature, inoculate it into a semi-solid medium containing tryptone beef infusion agar, and cultivate it at 33-37°C for 24-48 hours. Transfer to semi-solid medium, and cultivate at 33-37°C for 24-48 hours. Then transfer to the strain bottle containing tryptone beef extract liquid medium to carry out enrichment culture, and cultivate at 33-37 DEG C for 24-48 hours. The culture in the strain bottle is transferred to a fermenter of double peptone liquid medium, and cultured at 33-37° C. for 60-80 hours. After the fermentation is completed, formaldehyde with a final concentration of 0.1-0.5% is added for sterilization treatment, and the supernatant is collected by centrifugation. The supernatant was clarified and filtered through a 0.45-1.2 μm filter, and then concentrated by a 50-100KD membrane ultrafiltration. Add NaHCO separately 3 The final ...
Embodiment 3
[0236] Rabies Whole Virus Particle-Carrier Protein Immunogenic Conjugate Preparation and Vaccine Preparation:
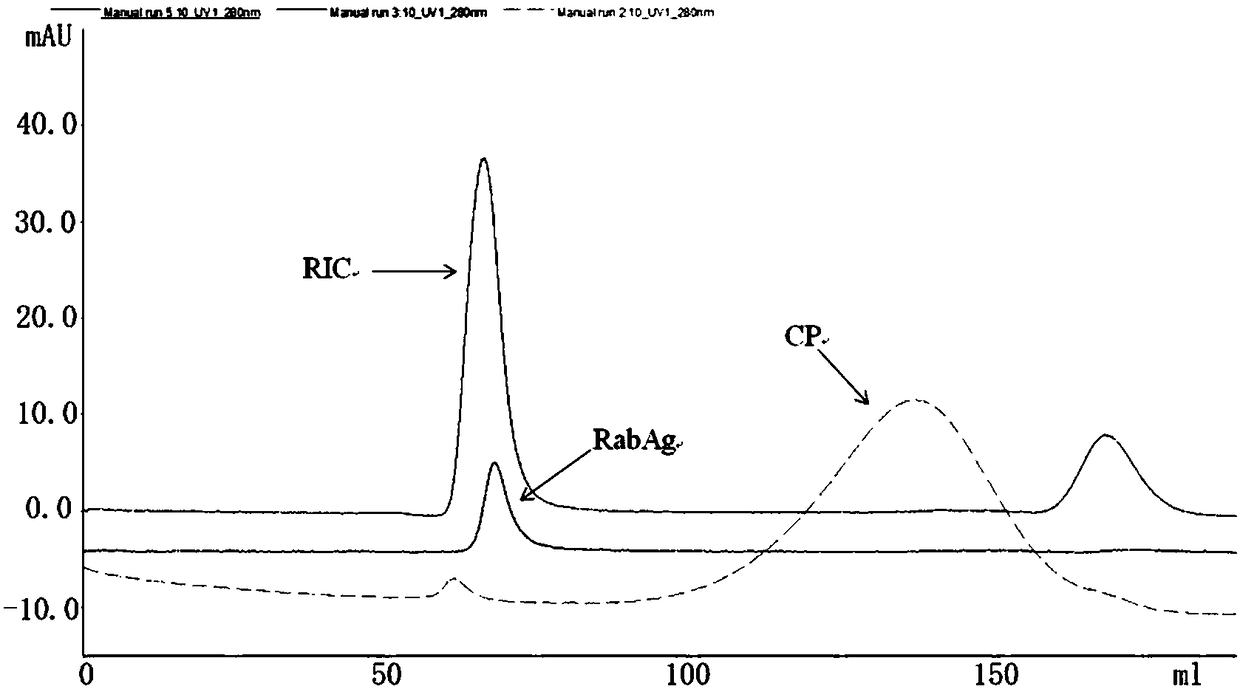
[0237] Measure the stock solution of tetanus toxoid and dilute it to 1-10 mg / ml with 0.1 M PBS with pH 7.5. Add 100mg / ml CDAP (1-cyano-4-dimethylamino-pyridine tetrafluoroboric acid) acetonitrile solution to a final concentration of 0.5-1.5mg / mgTT, react for 30 seconds, add 0.2M TEA (triethylamine), The ratio of TEA addition to CDAP addition is 1-3:1 (v / v), adjust the pH to 9.0, and maintain the reaction for 2-5 minutes. Add ADH (adipic hydrazide) NaHCO 3 (0.1M) solution to a final concentration of 2.0-4.0 mg / mgTT, and maintain the reaction for 120 minutes. Use 50-100KD membrane ultrafiltration to remove residual chemical reagents, which is the stock solution of tetanus toxoid‐ADH derivatives. Measure the purified stock solution of rabies whole virus particles prepared according to the method of Example 1 and without adding human serum albumin, according to the ra...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com