A kind of ferulic acid derivative and its application
A kind of derivative, the technology of ferulic acid, applied in synthetic chemicals, ferulic acid derivatives and their application fields, can solve the problem of no herbicide report and the like, achieve high and kill and remove weeds activity, preparation method Simple, high weed growth inhibitory effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
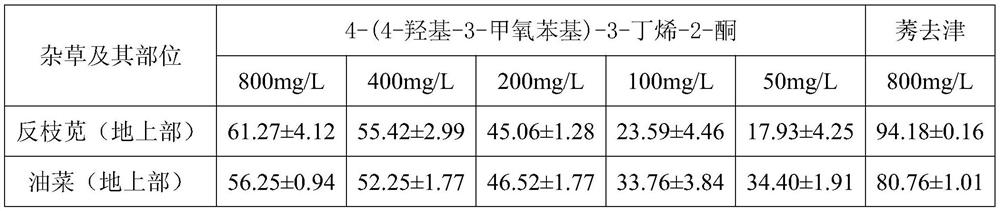
[0015] (1) Synthesis of 4-(4-hydroxy-3-methoxyphenyl)-3-buten-2-one
[0016] Add 7.6g (0.05mol) of vanillin and 100mL of acetone into a 250mL four-necked bottle with a thermometer and condensing reflux, put it into the rotor and stir to dissolve, then add 6mol / L HCl 10mL dropwise, react at room temperature for 24h, use in different time periods TLC monitored the entire reaction process. After the reaction, the reaction mixture was neutralized with 2.5mol / L NaOH 24mL, then 100mL of water was added, the reaction mixture was transferred to a 500mL separatory funnel, 200mL of ethyl acetate was added, and the organic layer was taken after shaking. with anhydrous MgSO 4 The organic layer was dried and concentrated in vacuo. Finally, the obtained crude product was purified by silica gel column chromatography, and the eluent was eluted with dichloromethane:methanol=20:1, and finally a yellow solid was obtained.
[0017] (2) Determination of herbicidal activity
[0018] The herbici...
Embodiment 2
[0028] (1) Synthesis of 4-hydroxy-3-methoxyvinyl cinnamate
[0029] Add 2.91g (0.015mol) of 4-hydroxy-3-methoxycinnamic acid, 0.35g (1.56mmol) of palladium diacetate, 0.56g (0.01 mol) and 100 mL of vinyl acetate, put into the rotor to stir and dissolve, react at room temperature for 10 h, and monitor the entire reaction process with TLC in different time periods. After the reaction, the reactant was filtered, washed three times with vinyl acetate, and evaporated in vacuo to obtain the crude product, which was purified by silica gel chromatography, and the eluent was gradient eluted with ethyl acetate:cyclohexane=5-30%, and collected product.
[0030] (2) Determination of herbicidal activity
[0031] The herbicidal activity of the compounds against several weeds was determined by the small cup method. The determination method is:
[0032] Take an appropriate amount of plump and undamaged weed seeds, soak and wash them with 1.5% sodium hypochlorite, rinse them with clean wat...
Embodiment 3
[0041] (1) Synthesis of 3-(4-hydroxyl-3-methoxyphenyl)-N-(4-methoxyphenyl)acrylamide
[0042] In an ice-water bath at 0° C., 100 mL of DMF was added to a 250 mL four-necked flask containing 10 mL of triethylamine, and 2.91 g (0.015 mol) of ferulic acid was added. Put into the rotor, stir to dissolve, then add 1.25mL p-methoxyaniline. Will use 30mLCH 2 Cl 2 Put 5 mmol of the dissolved Carter condensing agent into the constant pressure dropping funnel, complete the dropwise addition within 30 minutes, stir at room temperature, and react for 8 hours. The reaction mixture was diluted with a large amount of ethyl acetate, the mixture was washed with water and an aqueous solution of NaCl, and the organic layer was separated with a separatory funnel, and washed with anhydrous MgSO 4 dry. Concentrate by filtration to obtain a crude product, which is purified by silica gel column chromatography, and the eluent is eluted with ethyl acetate-petroleum ether, and finally the product is...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com