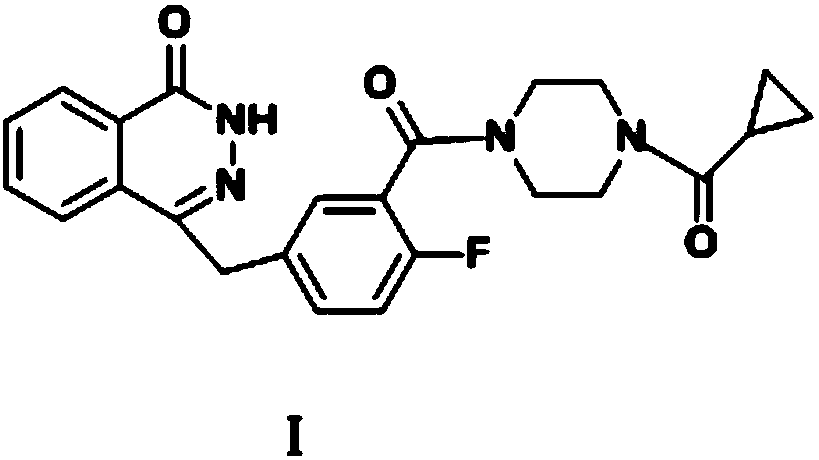
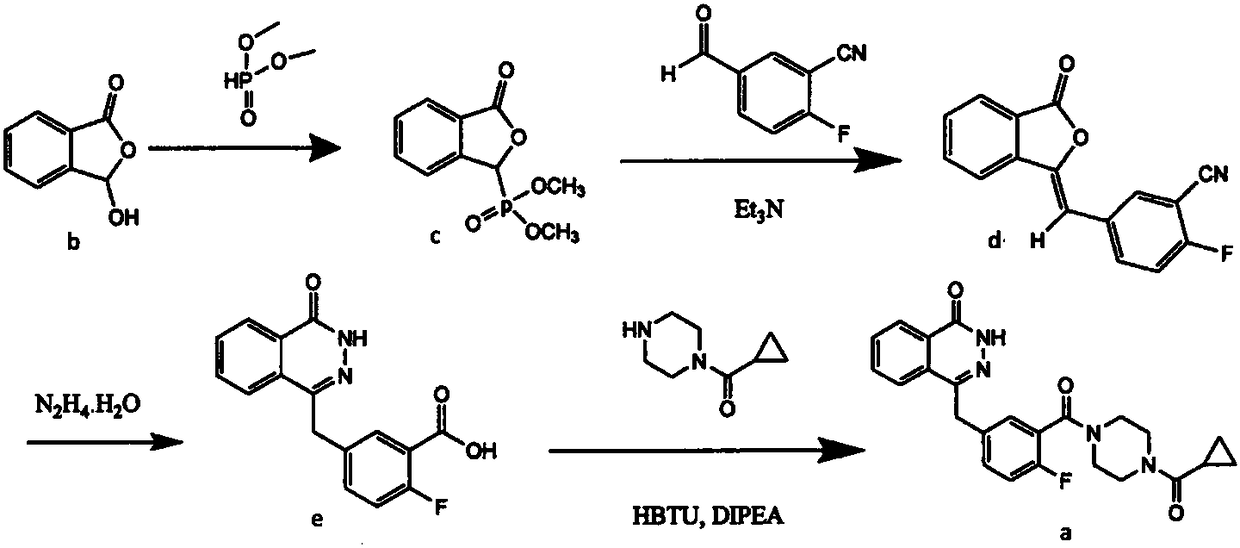
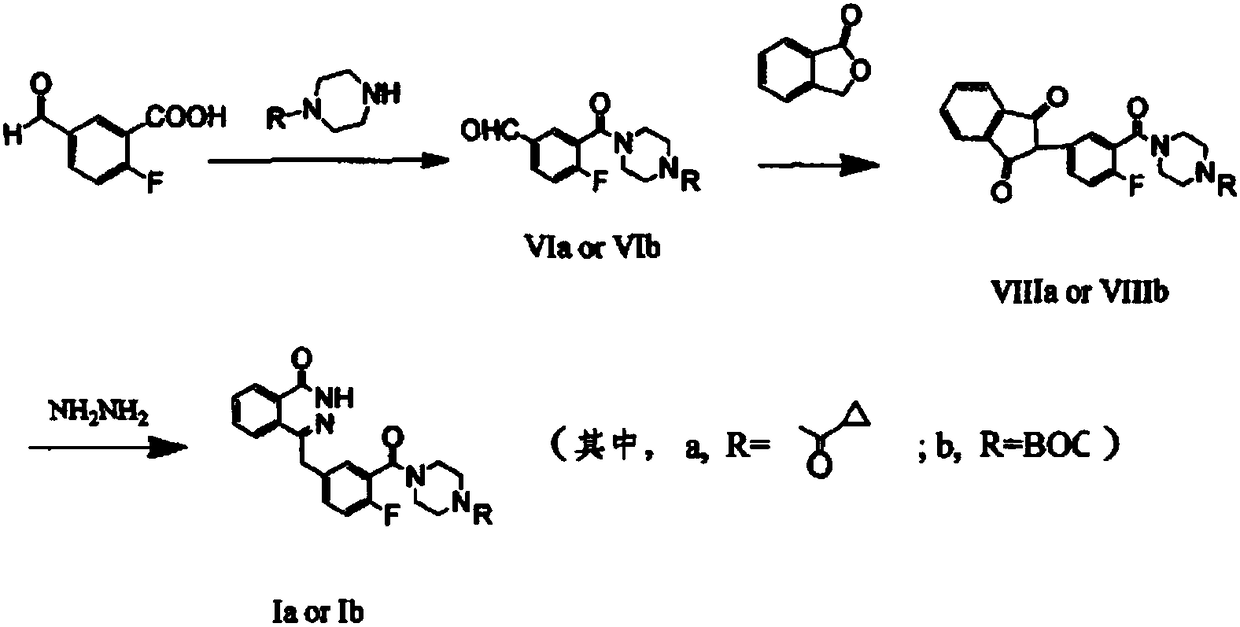
Preparation method of Olaparib
A technology of hydrazine monohydrate and compounds, which is applied in the field of preparation of the antineoplastic drug olaparib, can solve the problems of high raw material cost, low yield, long synthetic route, etc., and achieve product yield and purity improvement, and simple synthetic route , The effect of high total product yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Preparation of compound Ⅳ
[0031] Under nitrogen protection, add 2.4g of sodium hydride to the reaction flask and suspend in 150mL of dimethyl sulfoxide, stir at room temperature for 1h, add 13.4g of phthalide (II) and 20.3g of 3-bromo-4-fluorobenzaldehyde (III) , Continue stirring at room temperature for 2.5h to terminate the reaction. The reaction solution was dried under reduced pressure, and the residue was dissolved in 150 ml of ethyl acetate, washed with deionized water, dried over anhydrous magnesium sulfate, filtered, and the solvent was distilled off under reduced pressure to obtain 27.81 g of white crystals with a yield of 87%. HPLC The purity is 99.82%.
Embodiment 2
[0033] Preparation of compound Ⅳ
[0034] Under the protection of nitrogen, add 3.6g of sodium hydride to the reaction flask and suspend in 150mL of N,N-dimethylformamide, stir at room temperature for 1h, add 13.4g of phthalide (II) and 3-bromo-4-fluorobenzaldehyde ( Ⅲ) 18.27g, continue stirring at room temperature for 2.5h to terminate the reaction. The reaction solution was dried under reduced pressure, and the residue was dissolved in 150 ml of ethyl acetate, washed with deionized water, dried over anhydrous magnesium sulfate, filtered, and the solvent was distilled off under reduced pressure to obtain 25.87 g of white crystals. The yield was 90%, and the HPLC purity was 99.92%.
Embodiment 3
[0036] Preparation of compound Ⅳ
[0037] Under nitrogen protection, add 3.6g of sodium hydride and suspend in 150mL of tetrahydrofuran in the reaction flask, stir at room temperature for 1h, add 13.4g of phthalide (II) and 20.3g of 3-bromo-4-fluorobenzaldehyde (III), and continue stirring at room temperature 2.5h to terminate the reaction. The reaction solution was dried under reduced pressure, and the residue was dissolved in 150 ml of ethyl acetate, washed with deionized water, dried over anhydrous magnesium sulfate, filtered, and the solvent was distilled off under reduced pressure to obtain 29.37 g of white crystals. The yield was 92%, and the HPLC purity was 99.95%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com