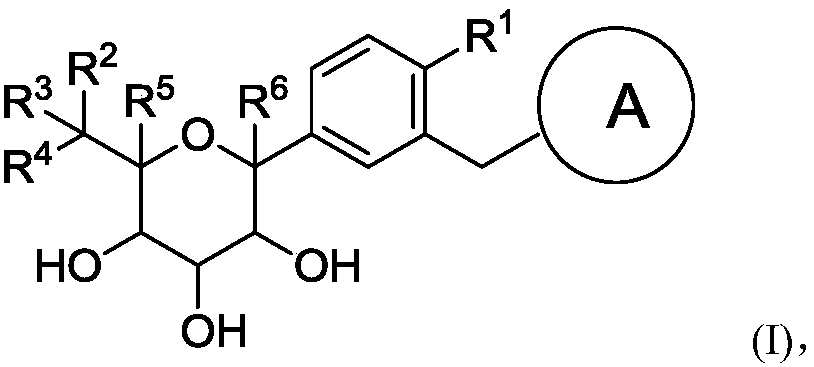
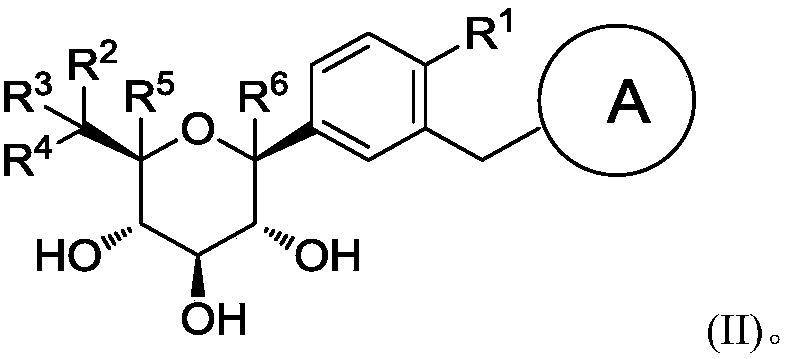
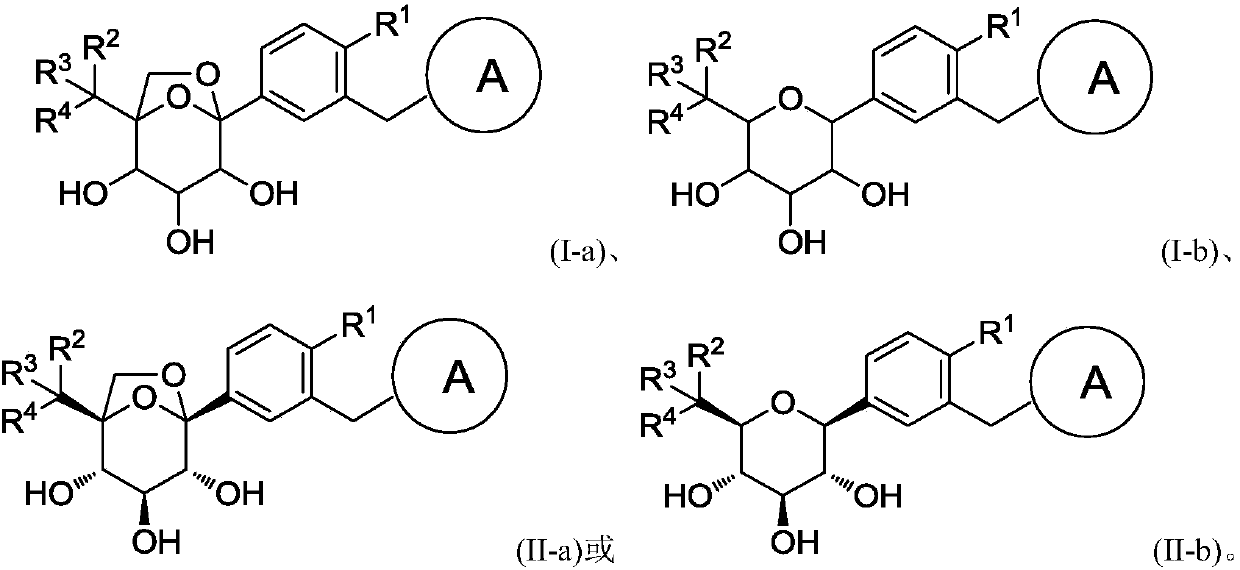
Glucopyranose-based derivative and medical application thereof
An alkyl and alkenyl technology, applied in the field of glucopyranosyl derivatives, can solve the problems of lactic acidosis, hypoglycemia, improper use of glinides, etc., and achieve excellent hypoglycemia, good pharmacological activity, and excellent SGLT2 inhibitory activity. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0268] Example 1 (1S, 2S, 3S, 4R, 5S)-5-[3-(2,3-dihydro-1,4-benzodioxin-6-ylmethyl)-4-fluoro-benzene Base]-1-(hydroxymethyl)-6,8-dioxobicyclo[3.2.1]octane-2,3,4-triol 1
[0269]
[0270] Step 1 5-Bromo-2-fluoro-benzoyl chloride 1b
[0271] N,N-Dimethylformamide (0.1 mL, 1.2 mmol) was added dropwise to a solution of 5-bromo-2-fluoro-benzoic acid 1a (1.0 g, 4.57 mmol) in thionyl chloride (10 mL) at room temperature , heated to 75°C and reacted for 4 hours. After the reaction, it was concentrated under reduced pressure to remove the organic solvent to obtain the title compound 1b (1.1 g, brown oil), yield: 100%. The obtained product was directly used in the next reaction.
[0272] Step 2 (5-Bromo-2-fluoro-phenyl)-(2,3-dihydro-1,4-benzodioxin-6-yl)methanone 1c
[0273] At room temperature, the crude 5-bromo-2-fluoro-benzoyl chloride 1b (1.1 g, 4.62 mmol) obtained above was dissolved in dichloromethane (10 mL) solution, and 2,3-dihydro-1,4- After benzodioxin (0.62g, 4.57...
Embodiment 2
[0302] Example 2 (1S, 2S, 3S, 4R, 5S)-5-[3-(2,3-dihydro-1,4-benzodioxin-6-ylmethyl)-4-fluoro-benzene Base]-1-(1-hydroxy-1-methyl-ethyl)-6,8-dioxobicyclo[3.2.1]octane-2,3,4-triol 2
[0303]
[0304]
[0305] Step 1 (1S,2S,3S,4R,5S)-2,3,4-tribenzyloxy-5-[3-(2,3-dihydro-1,4-benzodioxin-6- Methyl)-4-fluoro-phenyl]-6,8-dioxobicyclo[3.2.1]octane-1-carboxylic acid 2a
[0306] At 0°C, the Dess-Martin oxidant (0.56g, 1.3mmol) was added to [(1S,2S,3S,4R,5S)-2,3,4-tribenzyloxy-5-[3-(2 ,3-dihydro-1,4-benzodioxin-6-ylmethyl)-4-fluoro-phenyl]-6,8-dioxobicyclo[3.2.1]octane-1-yl] Methanol 1o (227 mg, 0.32 mmol) was dissolved in dichloromethane (10 mL), and the resulting mixture was heated to 40° C. and stirred for 16 hours. After the reaction, concentrate under reduced pressure, remove the organic solvent, add saturated sodium bicarbonate solution (30mL) to the residue, then extract with ethyl acetate (10mL×3), combine the organic phases, concentrate under reduced pressure, and pa...
Embodiment 3
[0315] Example 3 (1S, 2S, 3S, 4R, 5S)-5-[4-chloro-3-(2,3-dihydro-1,4-benzodioxin-6-ylmethyl)phenyl ]-1-(1-Hydroxy-1-methyl-ethyl)-6,8-dioxabicyclo[3.2.1]octane-2,3,4-triol 3
[0316]
[0317] Step 1 (2S,3R,4S,5R,6R)-2-[4-chloro-3-(2,3-dihydro-1,4-benzodioxin-6-ylmethyl)benzene base]-3,4,5-tris(trimethylsiloxy)-6-(trimethylsiloxymethyl)tetrahydropyran-2-ol 3b
[0318]Under nitrogen atmosphere, 6-[(5-bromo-2-chloro-phenyl)methyl]-2,3-dihydro-1,4-benzodioxin 3a (30.0g, 88.3mmol) was dissolved In anhydrous tetrahydrofuran (200mL), and cooled to -78 ° C, then slowly dropwise added n-butyllithium n-hexane solution (37mL, 89mol, 2.4M), after the dropwise addition was completed, stirred for 40 minutes, and then to the reaction Add (3R,4S,5R,6R)-3,4,5-tris(trimethylsilyloxy)-6-(trimethylsilyloxymethyl)tetrahydropyran-2- A solution of ketone 1f (37.8 g, 81.0 mmol) in anhydrous THF (400 mL) was stirred for 16 hours. After the reaction was completed, slowly drop water (5mL) to q...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com