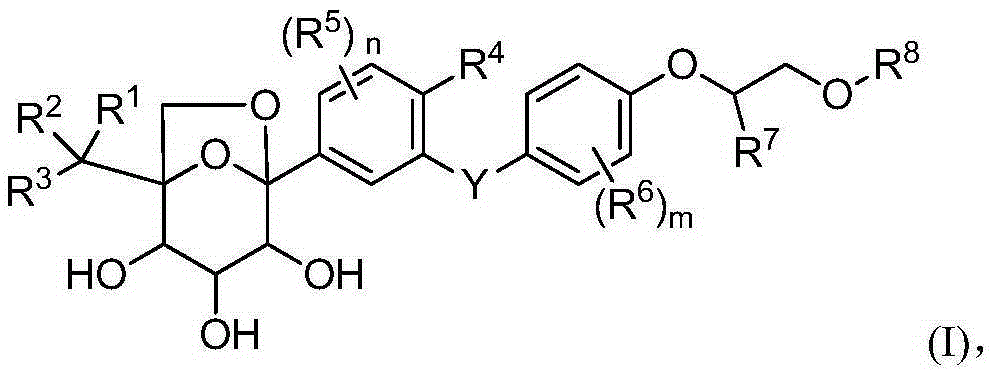
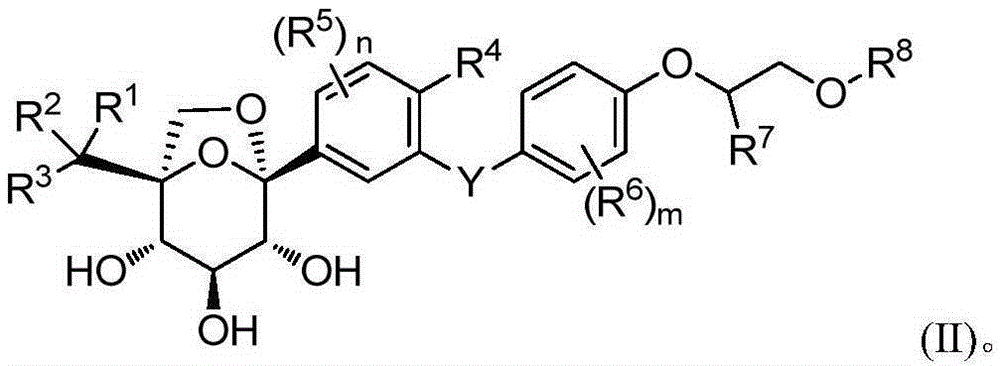
Glucopyranosyl derivative and application thereof in medicines
An alkyl and aryl technology, applied to glucopyranosyl derivatives and their application in medicine, can solve problems such as improper use of glinides, hypoglycemia, lactic acidosis, etc., and achieve good pharmacological activity, high Bioavailability, good absorption effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
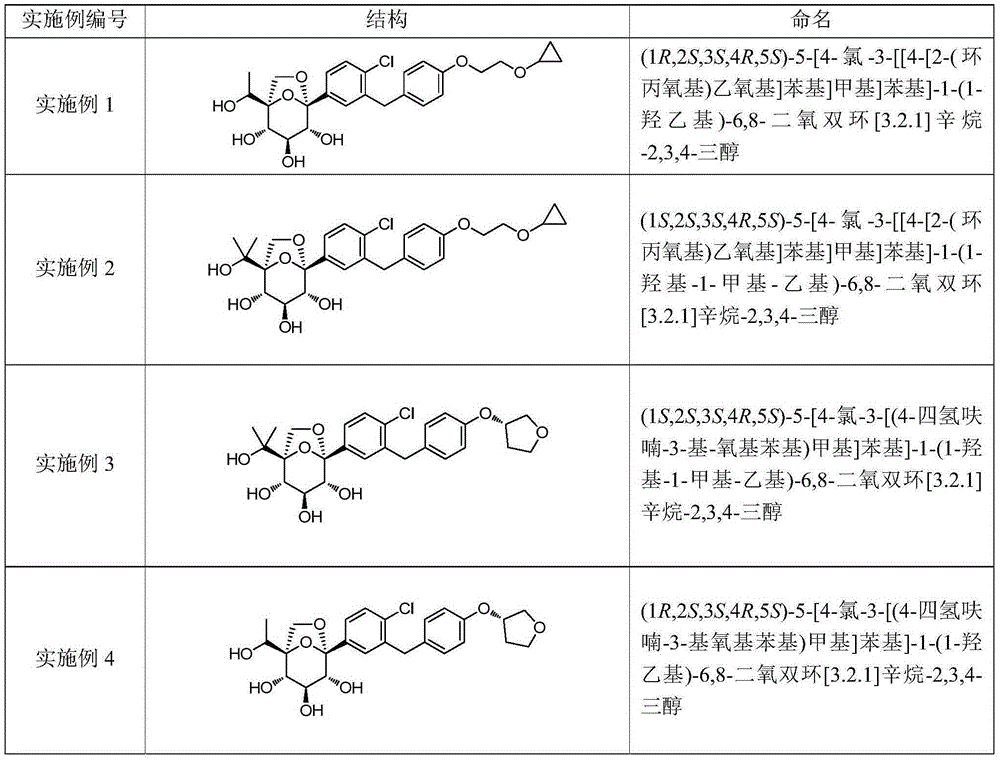
Embodiment 1
[0178] (1R,2S,3S,4R,5S)-5-[4-chloro-3-[[4-[2-(cyclopropoxy)ethoxy]phenyl]methyl]phenyl]-1- (1-Hydroxyethyl)-6,8-dioxbicyclo[3.2.1]octane-2,3,4-triol 1
[0179]
[0180]
[0181] step 1
[0182] 2-(Cyclopropoxy)ethanol 1b
[0183] At room temperature, the Mg (24.0g, 994.2mmol), I 2 (4.2 g, 16.6 mmol) was added to stirring tetrahydrofuran (200 mL). The temperature was raised to 45°C, and the solution of 1,2-dibromoethane (124.0g, 662.8mmol) in tetrahydrofuran (500mL), 2-(2-bromoethyl)-1,3-dioxolane 1a(30.0 g, 165.7 mmol) was added dropwise to the reaction solution, and stirring was continued at 45°C for 16 hours. After the reaction was completed, the reaction was quenched with 100 mL of saturated aqueous ammonium chloride solution, and the resulting mixture was filtered under reduced pressure with suction. The filtrate was washed with saturated brine (300 mL×3), dried over anhydrous sodium sulfate, filtered, and the filtrate was concentrated under reduced pressure to obtain the cru...
Embodiment 2
[0236] (1S,2S,3S,4R,5S)-5-[4-chloro-3-[[4-[2-(cyclopropoxy)ethoxy]phenyl]methyl]phenyl]-1- (1-hydroxy-1-methyl-ethyl)-6,8-dioxbicyclo[3.2.1]octane-2,3,4-triol 2
[0237]
[0238] step 1
[0239] (1S,2S,3S,4R,5S)-2,3,4-tribenzyloxy-5-[4-chloro-3-[[4-[2-(cyclopropoxy)ethoxy]benzene Yl]methyl]phenyl]-6,8-dioxbicyclo[3.2.1]octane-1-carboxylic acid 2a
[0240] At 0℃, add sodium bicarbonate aqueous solution (12mL, 8.65mmol, 0.72M), potassium bromide (18.7mg, 0.157mmol), tetramethylpiperidine (12.3mg, 0.078mmol) to [(1S, 2S ,3S,4R,5S)-2,3,4-tribenzyloxy-5-[4-chloro-3-[[4-[2-(cyclopropoxy)ethoxy]phenyl]methyl ]Phenyl]-6,8-dioxbicyclo[3.2.1]octan-1-yl]methanol 1p (0.60g, 0.786mmol, see Example 1, step 13) in tetrahydrofuran (12mL) solution, and then Sodium hypochlorite solution (10.8mL, 12.7mmol, effective chlorine content of 3.5%) was dropped into the reaction system, and the reaction solution was stirred at 0°C for 2 hours. After the reaction, 1M hydrochloric acid was added to adjust t...
Embodiment 3
[0255] (1S,2S,3S,4R,5S)-5-[4-chloro-3-[(4-tetrahydrofuran-3-yl-oxyphenyl)methyl]phenyl)-1-(1-hydroxy- 1-methyl-ethyl)-6,8-dioxbicyclo[3.2.1]octane-2,3,4-triol 3
[0256]
[0257]
[0258] step 1
[0259] (S)-Tetrahydrofuran-3-yl-4-methylbenzenesulfonate 3b
[0260] At room temperature, add p-toluenesulfonyl chloride (6.5g, 34.0mmol) to the dichloromethane solution of (S)-tetrahydrofuran-3-ol 3a (1.82mL, 22.7mmol) and imidazole (3.86g, 56.7mmol) ( 60 mL), a catalytic amount of 4-dimethylaminopyridine was added, and the resulting reaction system was stirred at room temperature for 16 hours. After the completion of the reaction, add 20 mL of water and 50 mL of dichloromethane to the reaction solution, stir and separate the layers. The separated organic phase was washed with saturated brine (20 mL×2), dried over anhydrous sodium sulfate, filtered, and the filtrate was concentrated under reduced pressure. The residue was purified by silica gel column chromatography [petroleum ether / eth...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com