A method for synthesizing sucralose-6-ethyl ester
A technology of sucralose and ethyl ester, which is applied in the field of preparation of synthetic sweetener intermediates, can solve the problems of high chlorination reagent, high toxicity or corrosiveness, and high cost of raw materials, achieves low toxicity, low cost, and reduced usage Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0014] Embodiment 1: A method for synthesizing sucralose-6-ethyl ester mentioned in the present invention comprises the following steps:
[0015] First, make 2,6-diisopropylphenylcarbene copper, by catalyst in situ synthesis method: in a container, add 0.01 mol 2,6-diisopropylphenylcarbene, 0.01 mol cuprous chloride and 10ml Anhydrous tetrahydrofuran, under nitrogen atmosphere, stir at room temperature for 12 hours, then add 0.03 mol granular sodium metal, stir at room temperature for 6 hours, distill off tetrahydrofuran under reduced pressure, extract the obtained residue with n-hexane, then distill off n-hexane under reduced pressure The resulting solid is 2,6-diisopropylphenylcarbene copper;
[0016] Next, in a 500ml autoclave, add sucrose-6-ethyl ester in N,N-dimethylformamide solution at room temperature (containing 38.4g of sucrose-6-ethyl ester, 260.0 g of N,N-dimethylformamide g), 0.35g of 2,6-diisopropylphenylcarbene copper synthesized in situ according to Example 1,...
Embodiment 2~7
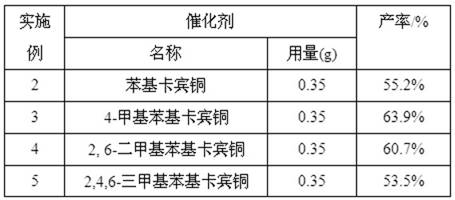
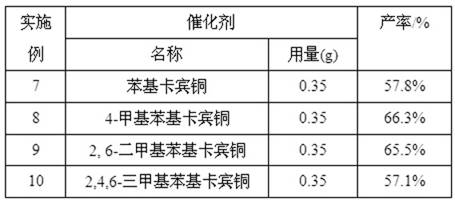
[0018] Method is similar to embodiment 2, adopts different catalysts to react, and the results are shown in Table 1:
[0019] Table 1 Catalytic reaction results of different catalysts (1)
[0020]
Embodiment 6
[0022] In a 500ml autoclave, add sucrose-6-ethyl ester in N,N-dimethylformamide solution at room temperature (containing 38.4g of sucrose-6-ethyl ester and 260.0g of N,N-dimethylformamide) , 0.35 g of 2,6-diisopropylphenylcarbene copper, 46.2 g of carbon tetrachloride, and 19.5 g of zinc powder synthesized in situ by the first step in Example 1. After the raw materials were added, the temperature was raised to 50° C., and the reaction was stirred for 3 hours. Then the temperature was raised to 80° C., and the reaction was stirred for 3 hours. Finally, the temperature was raised to 120° C., and the reaction was stirred for 3 hours. After the reaction, the reaction liquid contained 31.3 g of sucralose-6-ethyl ester (yield: 71.3%) through chromatography.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com