Polyimide resin containing furan ring and preparation method of polyimide resin
A technology of polyimide resin and furan ring, applied in the field of polyimide resin containing furan ring and preparation thereof, can solve the problems such as the glass transition temperature and thermal stability of polymers need to be further improved, and achieve high usage Effects of temperature and thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
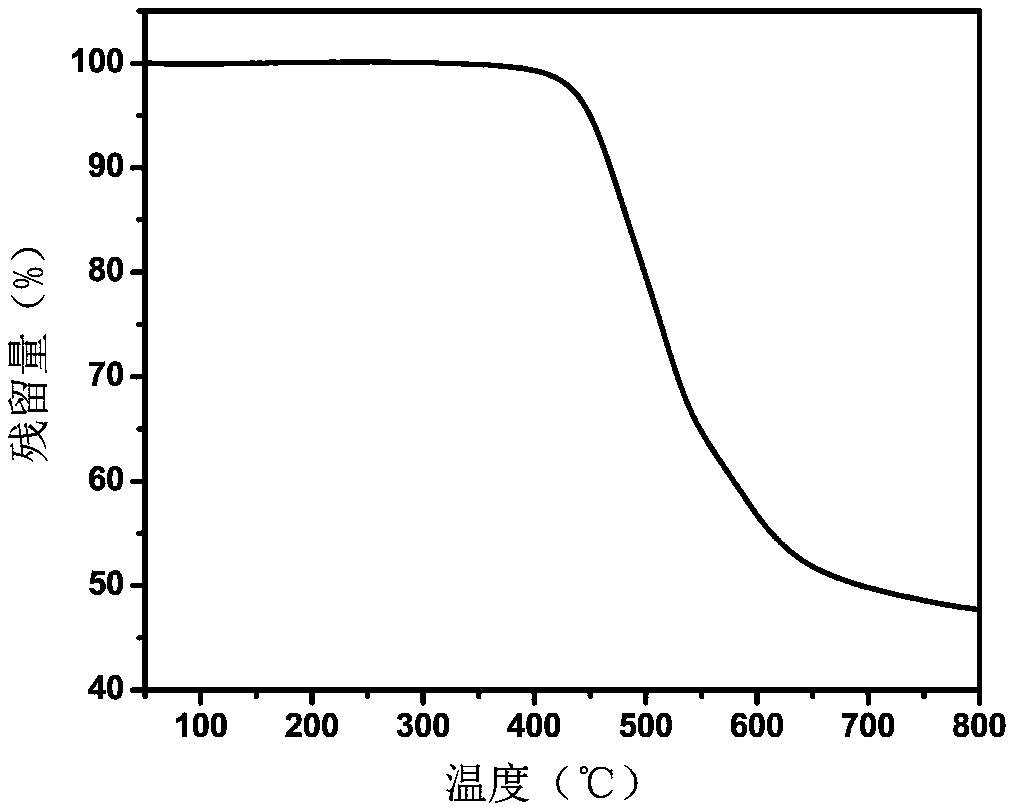
Embodiment 1
[0049] In this embodiment, the polyimide resin containing furan ring has the following structural formula:
[0050] Wherein, n is an integer greater than zero.
[0051] The specific preparation method is: under the protection of nitrogen, add 1.6817g (4.0mmol) of 2,5-furandi(formamide-p-phenylenediamine) and 16ml of N,N-dimethylacetamide into a 50ml reaction flask at room temperature After stirring, after 2,5-furandi(formamide-p-phenylenediamine) is completely dissolved, add 2.0805g (4.0mmol) of bisphenol A type diether dianhydride and continue stirring at room temperature for 24 hours to obtain a viscous Polyamic acid solution. The solution was then diluted to a concentration of 10% by weight. Then, a mixed solution of 4 mL of acetic anhydride and 2 mL of pyridine was added, and stirring was continued at room temperature for 24 hours to obtain a polyimide solution. Then, the polyimide solution was dropped into ethanol to obtain fibrous polyimide precipitation, which was dried ...
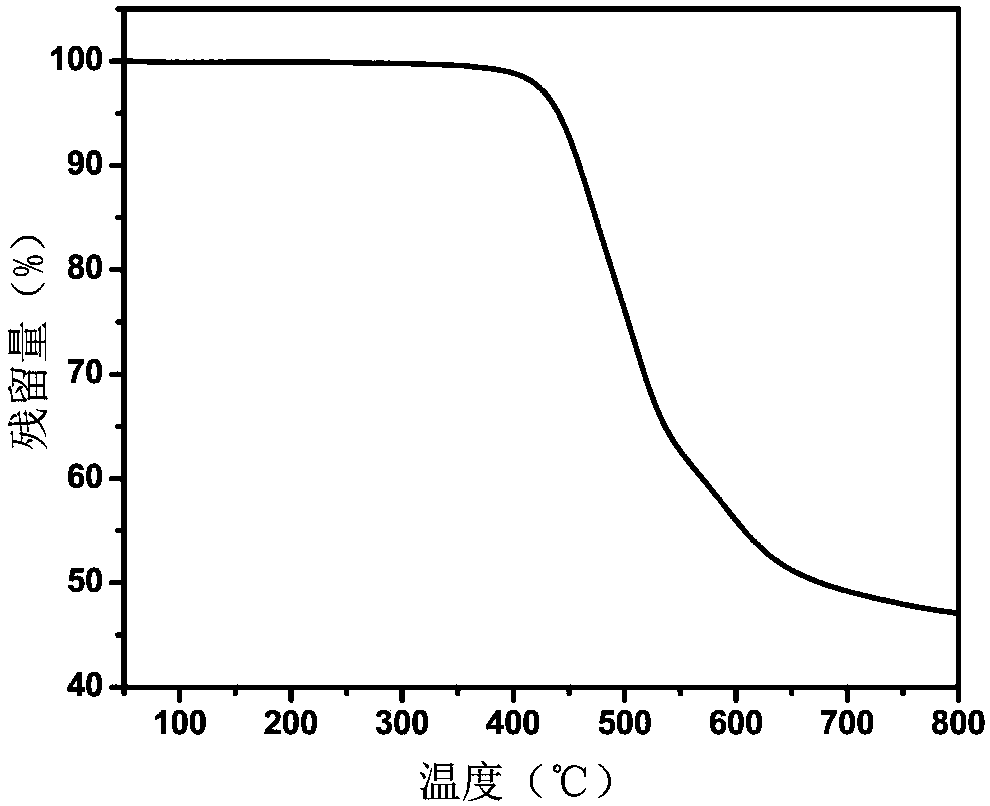
Embodiment 2
[0061] In this embodiment, the polyimide resin containing furan ring has the following structural formula:
[0062] Wherein, n is an integer greater than zero.
[0063] The specific preparation method is: under the protection of nitrogen, add 1.6817g (4.0mmol) of 2,5-furandi(m-phenylenediamine formamide) and 16ml of N,N-dimethylacetamide into a 50ml reaction flask at room temperature. After stirring, after 2,5-furandi(m-phenylenediamine formamide) is completely dissolved, add 2.0805g (4.0mmol) of bisphenol A type diether dianhydride and continue to stir at room temperature for 24 hours to obtain a viscous Polyamic acid solution, and then dilute the solution to a concentration of 10% by weight. Then, a mixed solution of 4 mL of acetic anhydride and 2 mL of pyridine was added, and stirring was continued at room temperature for 24 hours to obtain a polyimide solution. The polyimide solution was dropped into ethanol to obtain a fibrous polyimide precipitate, which was dried to obtai...
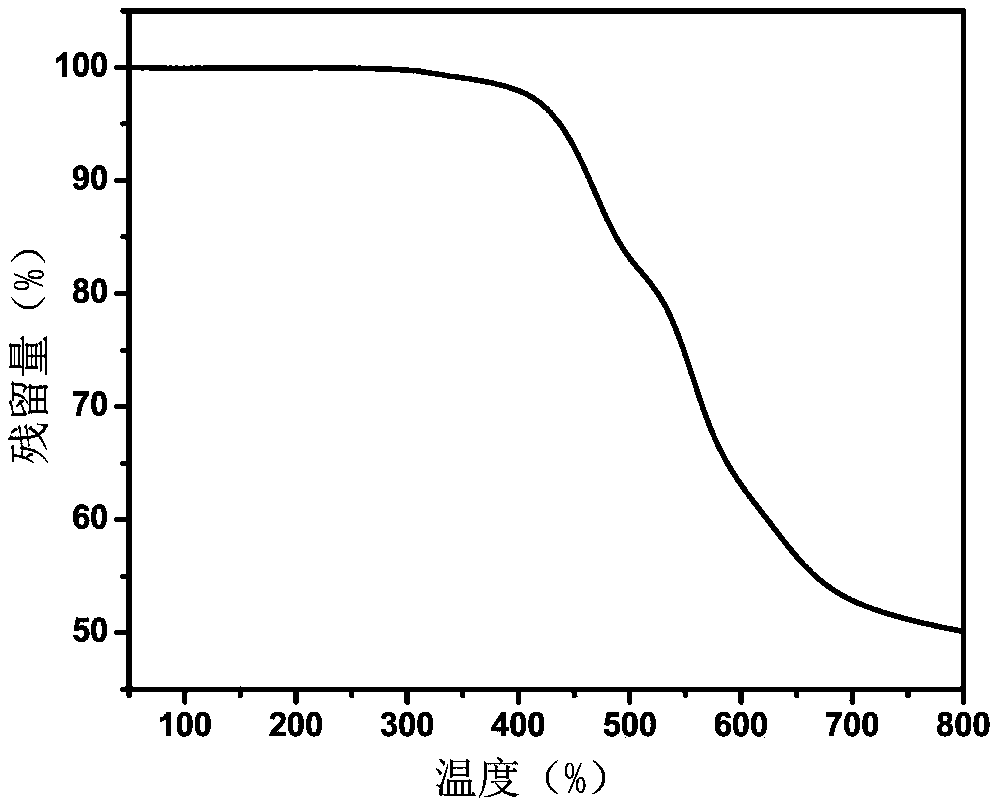
Embodiment 3
[0067] In this embodiment, the polyimide resin containing furan ring has the following structural formula:
[0068] Wherein, n is an integer greater than zero.
[0069] The specific preparation method is: under the protection of nitrogen, add 1.6817g (4.0mmol) of 2,5-furandi(formamide-p-phenylenediamine) and 16ml of N,N-dimethylacetamide into a 50ml reaction flask at room temperature Stir, after 2,5-furandi(formamide-p-phenylenediamine) is completely dissolved, add 1.7760g (4.0mmol) of 4,4'-(hexafluoroisopropylene) diphthalic anhydride, and continue to stir at room temperature for 24 hours , A viscous polyamic acid solution is prepared, and then the solution is diluted to a weight percentage of 10%. Then, a mixed solution of 4 mL of acetic anhydride and 2 mL of pyridine was added, and stirring was continued at room temperature for 24 hours to obtain a polyimide solution. The polyimide solution was dropped into ethanol to obtain a fibrous polyimide precipitate, which was dried to...
PUM
| Property | Measurement | Unit |
|---|---|---|
| glass transition temperature | aaaaa | aaaaa |
| tensile strength | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com