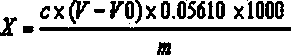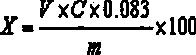Method of quickly measuring content of terephthalic acid
A technique for rapid determination of terephthalic acid, applied in the field of chemical analysis, to achieve the effect of shortening the analysis time, simple, fast and accurate operation, and expanding the test range
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Test the analytically pure AR of Guohua Reagent respectively with the method for measuring the acid value of purified terephthalic acid in the petrochemical industry standard "SH / T 1612.2-1995" and the method of the present invention's "Rapid Determination of Terephthalic Acid Content". A sample of phthalic acid.
[0022] The specific steps for the determination of the acid value of industrial purified terephthalic acid in the petrochemical industry standard "SH / T 1612.2-1995" are as follows:
[0023] Weigh about 1.0g of the sample to be tested (accurate to 0.0001g) into a 250mL Erlenmeyer flask. Add 20 mL of pyridine to the Erlenmeyer flask, stir until the PTA is basically dissolved, then add 20 mL of boiled and cooled distilled water, and continue stirring until the sample is completely dissolved. Add 0.1 mL of phenolphthalein indicator solution. Titrate with sodium hydroxide standard titration solution until it turns pink, and record the volume of standard titratio...
Embodiment 2
[0045] Use the method of "Rapid Determination of Terephthalic Acid Content" to determine the content of terephthalic acid in the terephthalic acid waste residue provided by Wujiang Aifeihua Textile Trading Co., Ltd. This sample is a tide product. The specific steps are as follows:
[0046] A. Accurately weigh about 2.0g of sample, accurate to 0.0001g, put it in a 250mL conical flask, add 1 drop of Span 80 (about 0.045g), add 20mL of water, shake well, sonicate until uniform, add 3 drops Phenolphthalein indicator solution, titrated with sodium hydroxide standard solution until the solution turns pink and keeps 30s without fading, which is the end point. The calculation method is the same as the calculation formula of the quick method in Example 1.
[0047] B. Accurately weigh about 3.0g of sample, accurate to 0.0001g, put it in a 250mL conical flask, add 2 drops of AEO-9 (about 0.09g), add 20mL of water, shake well, sonicate until uniform, add 3 drops Phenolphthalein indicator...
Embodiment 3
[0051]Use the method of "Quick Determination of Terephthalic Acid Content" to determine the content of terephthalic acid in the terephthalic acid waste residue provided by Yixing China Resources Materials Co., Ltd. This sample is a tide product. The specific steps are as follows:
[0052] D. Accurately weigh about 4.0g sample, accurate to 0.0001g, put it in a 250mL Erlenmeyer flask, add 1 drop of Tween 80 and 1 drop of Span 80, add 40mL of water, shake well, sonicate until uniform, add 3 Drop phenolphthalein indicator solution, titrate with sodium hydroxide standard solution until the solution turns pink, and keep it for 30s without fading, which is the end point. The calculation method is the same as the calculation formula of the fast method in Example 1.
[0053] E. Accurately weigh about 3.0g sample, accurate to 0.0001g, put it in a 250mL Erlenmeyer flask, add 1 drop of AEO-9 and 1 drop of OP-10, add 30mL of water, shake well, sonicate until uniform, add 3 Drop phenolphth...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com