A recombinant tuberculosis vaccine, its preparation method and application
A technology of recombinant virus vector and vaccine composition, which is applied in the fields of bioengineering and immunization, and can solve problems such as inability to apply to immunodeficiency patients, great changes in tuberculosis protection ability, and insufficient immune effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
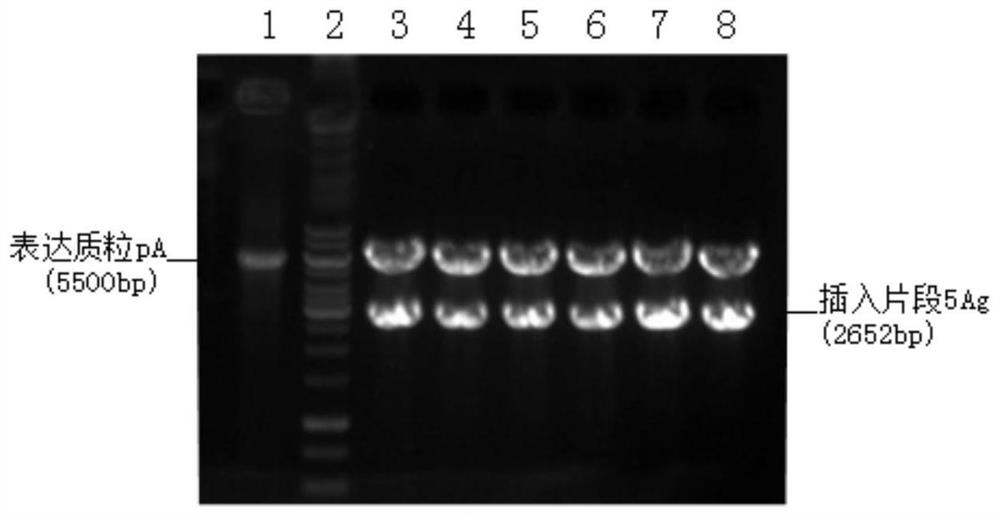
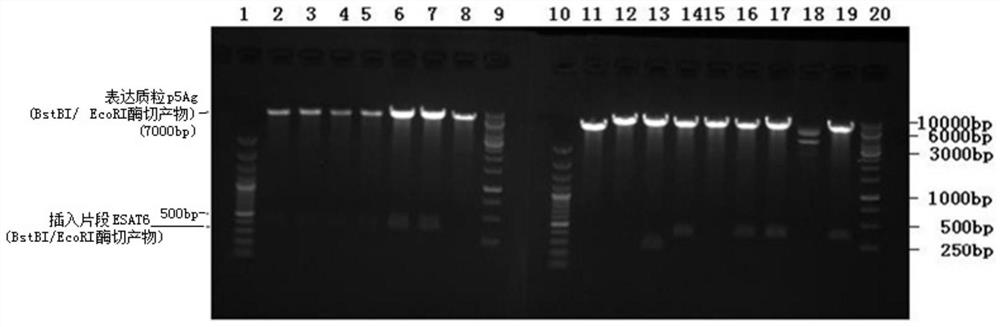
[0085] Embodiment 1, p5Ag (Kozak-TPA-pAg85B-ESAT6-Rv1733c-Rv2626c-RpfD) recombinant plasmid construction
[0086] In this example, the target fragment was inserted into the expression plasmid to construct the recombinant plasmid p5Ag (pKozak-TPA-Ag85B-ESAT6-Rv1733c-Rv2626c-RpfD).
[0087] 1.1 Obtain the digested vector
[0088] Prepare the enzyme digestion system as shown in Table 1.
[0089] Table 1 (μl)
[0090] Sample name expression plasmid pA 10×O buffer EcoRI SalI wxya 2 o
carrier 20 4.5 1 1 46
[0091] After mixing, react at 37°C for 2 hours. The digested product was purified with the nucleic acid gel recovery kit TIANgel MidiPurification Kit to obtain 40 μl of the product.
[0092] 1.2 Obtain the insert fragment after enzyme digestion
[0093] Prepare the enzyme digestion system as shown in Table 2.
[0094] Table 2 (μl)
[0095]
[0096] After mixing, react at 37°C for 1 hour. The digested product was purified with the ...
Embodiment 2
[0112] Embodiment 2, construction of p4Ag (pKozak-TPA-ESAT6-Rv1733c-Rv2626c-RpfD) recombinant plasmid
[0113] In this example, the target fragment was inserted into the expression plasmid to construct the recombinant plasmid p4Ag (pESAT6-Rv1733c-Rv2626c-RpfD).
[0114] 1. Obtain and amplify the Kozak-TPA-ESAT6 (370bp) fragment by PCR
[0115] 4Ag forward primer (SEQ ID NO: 7): cgaattcgccaccatggacgccatgaagaggggcctgtgttgcgtgctgctcctgtgtggcgccgtgttcgtgagccccagcaccgagcagcagtgga
[0116] 4Ag reverse primer (SEQ ID NO: 8): gcttcgaaggcgaacatgccggtcaca
[0117] Template: p5Ag (pAg85B-ESAT6-Rv1733c-Rv2626c-RpfD) or Mycobacterium tuberculosis H37Rv genome.
[0118] The reaction system is shown in Table 5.
[0119] Table 5. PCR reaction system (μl)
[0120]
[0121] PCR reaction conditions: 95°C for 3 minutes → (95°C for 30 seconds → 70°C for 30 seconds → 72°C for 60 seconds) 30 cycles → 72°C for 10 minutes.
[0122] Recovery of PCR products: PCR products were purified with TIAN...
Embodiment 3
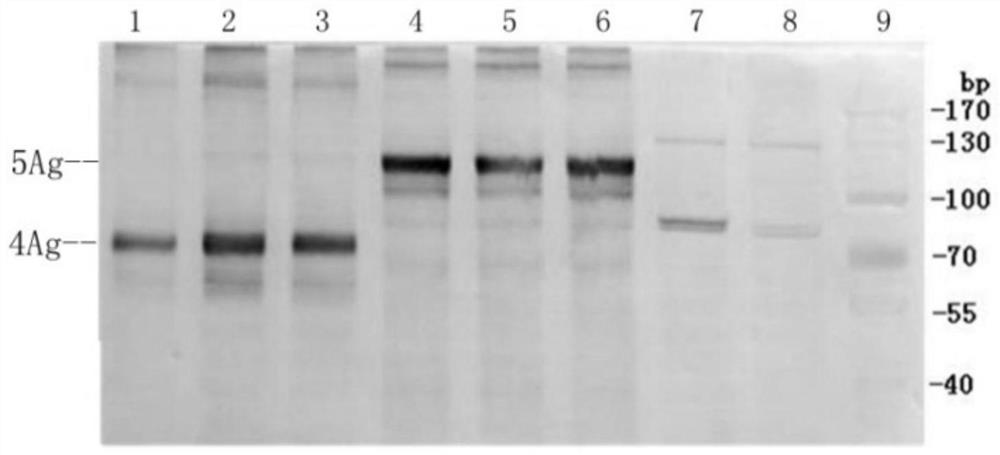
[0146] Embodiment 3, acquisition, amplification and identification of recombinant viral particles
[0147] In this example, the two recombinant expression plasmids prepared in Example 1-2 were introduced into vaccinia virus MVA by homologous recombination.
[0148] 1. Recombinant expression plasmid transfected with vaccinia virus
[0149] 1.1 Prepare BHK21 cells in logarithmic growth phase and infect 0.1 PFU of vaccinia virus MVA strain.
[0150] 1.2 One hour after MVA infection, remove the virus liquid, replace with new double-antibody-free medium and incubate at 37°C for 2 hours, and transfect the above two recombinant expression plasmids respectively.
[0151] 1.3 Harvest the virus after culturing at 37°C for 3 days, and freeze and thaw repeatedly 3 times.
[0152] 1.4 Re-infect BHK21 cells with the frozen-thawed cell lysate, and culture with MPA selection medium at 37°C for 2-5 days.
[0153] 1.5 Pick a single viral plaque, amplify it, and identify the picked clones. T...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com