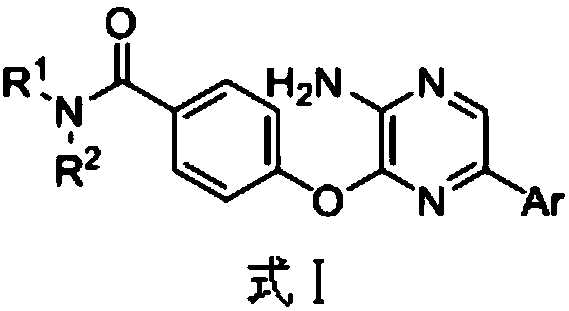
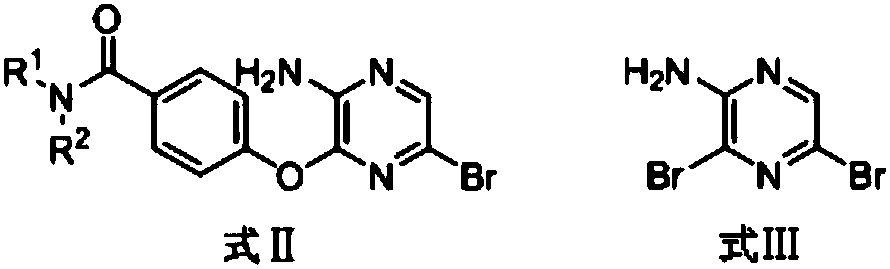
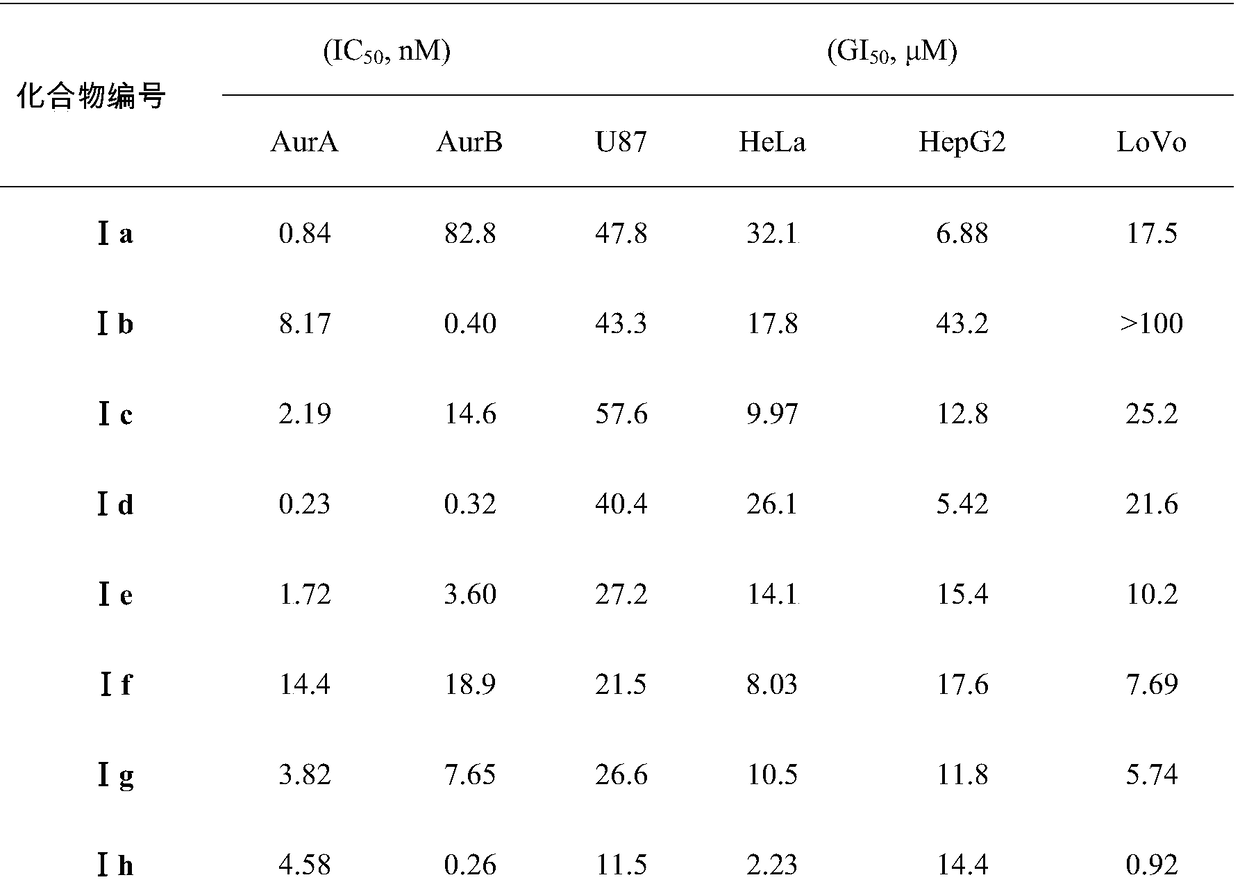
3,5-disubsitutued 2-amino-pyrazine compound and preparation technology and application thereof
A preparation process and compound technology, applied in organic chemistry, drug combination, antitumor drugs, etc., can solve the problem of no Aurora kinase inhibitor, etc., and achieve the effect of good proliferation inhibitory activity, strong inhibitory activity, and obvious inhibitory activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Embodiment 1: the preparation of p-hydroxybenzoic acid n-propionamide
[0024] Stir and dissolve p-hydroxybenzoic acid (690mg, 5.0mmol) in 15mL of acetone, then add n-propylamine (6.0mmol), and after complete dissolution, add 1-(3-dimethylaminopropyl)-3-ethylcarbodi Imine hydrochloride was refluxed at 70°C for 12h. After the reaction was finished, the acetone was distilled off, and the residue was diluted with ethyl acetate, and then washed with 1mol / L hydrochloric acid and 10wt% Na 2 CO 3 After washing, the organic phase was dried and the solvent was evaporated, and a white powder was obtained by column chromatography. The product detection data are as follows:
[0025] Yield: 71%; 1 H NMR (600MHz, DMSO-d 6 )δ10.08(s,1H),9.97(s,1H),7.85(d,J=9.0Hz,2H),7.75(d,J=7.2Hz,2H),7.32(t,J=7.8Hz, 2H), 7.07-7.06 (m, 1H), 6.86 (d, J=9.0Hz, 2H).
[0026] Use cyclopropylamine, cyclopentylamine, cyclohexylamine, morpholine, 4-methylpiperazine, aniline, p-chloroaniline or m-chlor...
Embodiment 2
[0027] Example 2: Preparation of 4-O-(3-amino-6-bromo-pyrazinyl-2)-benzoic acid n-propylamide
[0028] Synthetic general method: Dissolve 2-amino-3,5-dibromo-pyrazine (253mg, 1.0mmol) in 10mL N-methylpyrrolidone (or N-ethylpyrrolidone, or N-cyclohexylpyrrolidone), and then Add the p-hydroxybenzoic acid n-propionamide (1.2mmol) prepared above, and add K after completely dissolving 2 CO 3 (276mg, 2.0mmol), in N 2 Under protection, react at 100°C for 6h. After the reaction was completed, it was diluted with 30 mL of ethyl acetate, the organic layer was washed with saturated brine, dried, the solvent was evaporated and the column chromatography was used to obtain the intermediate 4-O-(3-amino-6-bromo-pyrazinyl-2)- N-Propylamide Benzoate. Bright brown liquid. The product detection data are as follows:
[0029] Yield: 90%; 1 H NMR (600MHz, DMSO-d 6 )δ9.94(s,1H),8.20(s,1H),7.70(d,J=8.4Hz,2H),6.78(d,J=8.4Hz,2H),3.19-3.15(m,2H), 1.52-1.48 (m, 2H), 0.87 (t, J = 7.2Hz, 3H).
[...
Embodiment 3
[0031] Example 3: Preparation of 4-O-(3-amino-6-(3,5-dimethylisoxazolyl-4)pyrazinyl-2)-benzoic acid n-propylamide
[0032] Get the 4-O-(3-amino-6-bromo-pyrazinyl-2)-benzoic acid n-propylamide (0.5mmol) and 3,5-dimethylisoxazole-4-boronic acid ( 84.6mg, 0.6mmol) was dissolved in 10mL 1,4-dioxane, and then the catalyst Pd(dppf)Cl was added 2 ·CH 2 Cl 2 (40.8mg, 0.05mmol), 2mol / L Na 2 CO 3 solution (0.05mL, 0.1mmol), in N 2 Reflux at 100°C under protection until the reaction is complete, evaporate the solvent under reduced pressure, and dilute the residue with 15 mL of ethyl acetate, wash with saturated brine, dry, and evaporate the solvent to obtain a white solid by column chromatography. The product detection data are as follows:
[0033] Yield: 72%; Purity: 95% (HPLC:MeOH:H 2 O=70:30,0.5mL / min,t R =7.60min); 1 HNMR (600MHz, DMSO-d 6 )δ8.44(t, J=5.4,1H),7.89(d,J=7.2Hz,2H),7.79(s,1H),7.31(d,J=7.2Hz,2H),6.78(s,2H ),3.21-3.18(m,2H),2.29(s,3H),2.07(s,3H),1.53-1.49(m,2H),...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com