A kind of magnetic sulfonic acid functionalized cofs material and its preparation method and application
A sulfonic acid-based, functionalized technology, applied in chemical instruments and methods, separation methods, analytical materials, etc., can solve the problem of poor selective adsorption performance of aromatic amine compounds, and achieve strong electrostatic adsorption and efficient adsorption effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
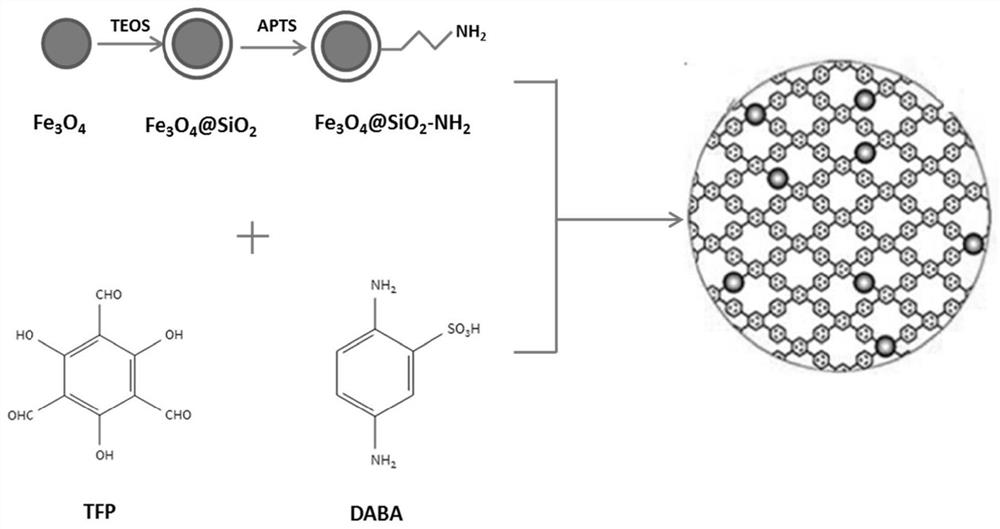
[0040] The magnetic sulfonic acid functionalized COFs material of this embodiment, such as figure 1 As shown, the following steps are used to prepare:
[0041] Dissolve 2,4,6-triformylpyroglucinol (21.0mg), 2,5-diaminobenzenesulfonic acid (28.2mg) in trimethylbenzene / dioxane mixed solvent (from 0.2mL Trimethylbenzene, 0.8mL dioxane mixed), add 0.1mL acetic acid solution with a concentration of 3mol / L, mix at room temperature to form a homogeneous solution; then add 0.02g magnetic Nanoparticles were reacted at 100°C for 72 hours. After the reaction, a black solid product was isolated, washed successively with anhydrous dimethylformamide, anhydrous tetrahydrofuran and anhydrous acetone, and washed 2-3 times with anhydrous methanol to complete the activation. Vacuum drying at 150°C for 12 hours yielded the magnetic sulfonic acid functionalized COFs material.
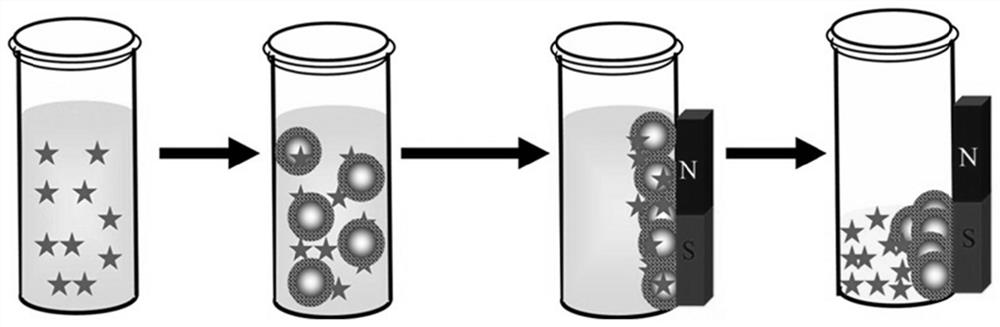
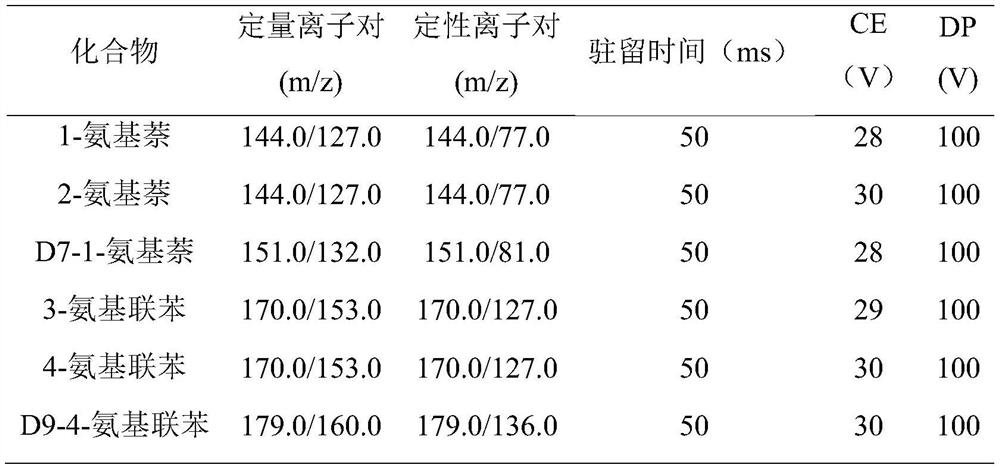
[0042] Applying the magnetic sulfonic acid functionalized COFs material of this embodiment to the detection of aromatic...
Embodiment 2
[0062] The magnetic sulfonic acid functionalized COFs material of this example is prepared by the following steps:
[0063] Dissolve 2,4,6-triformylpyroglucinol (11.0mg), 2,5-diaminobenzenesulfonic acid (18.2mg) in trimethylbenzene / dioxane mixed solvent (from 0.2mL Trimethylbenzene, 0.6mL dioxane mixed), add 0.1mL acetic acid solution with a concentration of 3mol / L, mix at room temperature to form a homogeneous solution; then add 0.02g magnetic Nanoparticles were reacted at 90°C for 80 hours. After the reaction, a black solid product was isolated, washed successively with anhydrous dimethylformamide, anhydrous tetrahydrofuran and anhydrous acetone, and then washed 2-3 times with anhydrous methanol to complete the activation. Vacuum drying at 150°C for 12 hours yielded the magnetic sulfonic acid functionalized COFs material.
Embodiment 3
[0065] The magnetic sulfonic acid functionalized COFs material of this example is prepared by the following steps:
[0066] Dissolve 2,4,6-triformylpyroglucinol (28.0mg), 2,5-diaminobenzenesulfonic acid (40.0mg) in trimethylbenzene / dioxane mixed solvent (from 0.2mL Trimethylbenzene, 1.2mL dioxane mixed), add 0.1mL acetic acid solution with a concentration of 3mol / L, mix at room temperature to form a homogeneous solution; then add 0.04g magnetic Nanoparticles were reacted at 120°C for 60 hours. After the reaction, a black solid product was isolated, washed successively with anhydrous dimethylformamide, anhydrous tetrahydrofuran and anhydrous acetone, and washed 2-3 times with anhydrous methanol to complete the activation. Vacuum drying at 150°C for 12 hours yielded the magnetic sulfonic acid functionalized COFs material.
[0067] In other embodiments of the magnetic sulfonic acid functionalized COFs material of the present invention, the amount of 2,4,6-triformylpyroglucinol, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com