Preparation and application of recombinant yeast preparation fused with bovine antimicrobial peptide fbap
A technology of recombinant bacteria and nucleic acid molecules, used in fusion polypeptides, hybrid peptides, antibacterial drugs, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
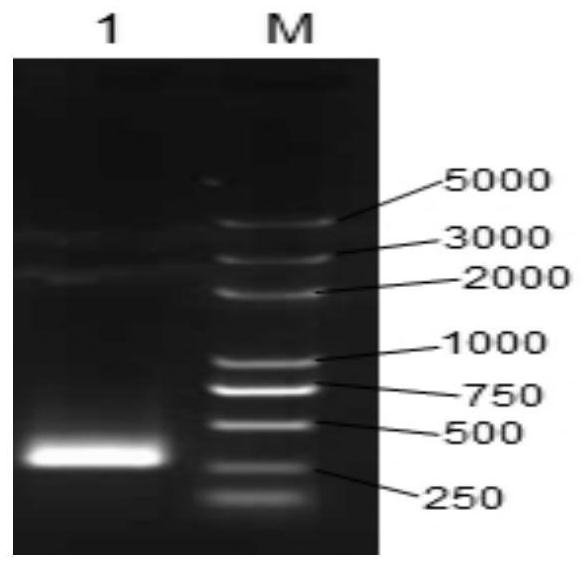
[0120] Embodiment 1, bovine antimicrobial peptide fusion protein (FBAP) and its coding gene
[0121] 1. Obtaining the fusion protein FBAP and its coding gene
[0122] Four kinds of bovine antimicrobial peptides were fused by connecting peptides to obtain a fusion protein named FBAP.
[0123] The amino acid sequence of the fusion protein is shown as sequence 1 in the sequence listing, the fusion gene encoding the fusion protein FBAP is named FBAP, and the nucleotide sequence of the fusion gene is sequence 2.
[0124] Among them, the 1st-12th position of sequence 1 is bovine antimicrobial peptide F, the 13th-20th position is a linking peptide, the 21st-32nd position is bovine antimicrobial peptide B, the 33rd-40th position is a linking peptide, and the 41st-55th position is Bovine antimicrobial peptide A, the 56th-62nd is the connecting peptide, and the 63rd-75th is the bovine antimicrobial peptide P.
[0125] The 1st-36th position of sequence 2 is the nucleic acid encoding bo...
Embodiment 2
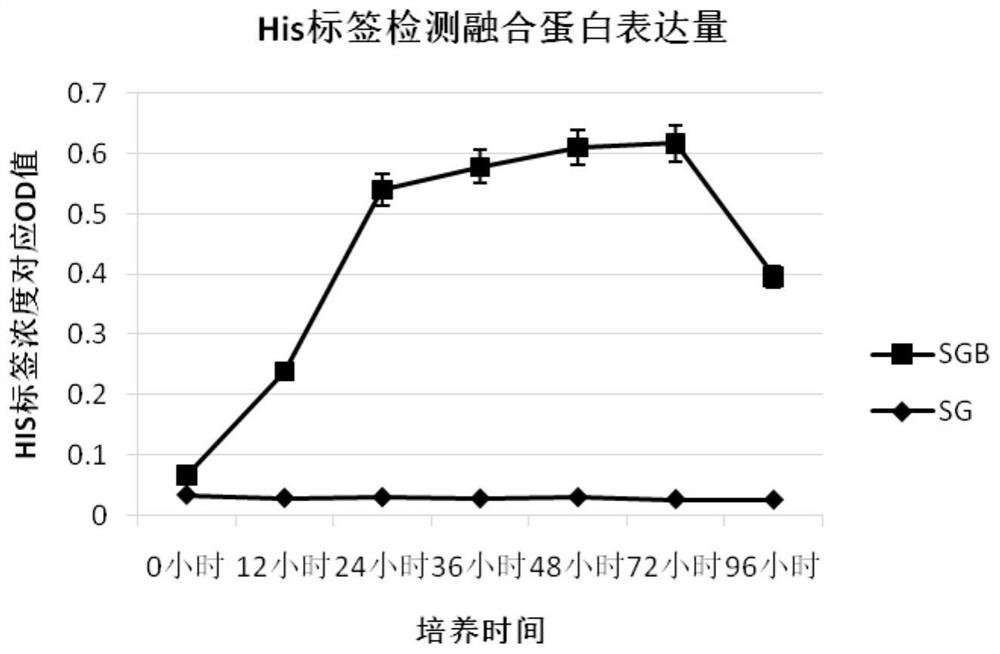
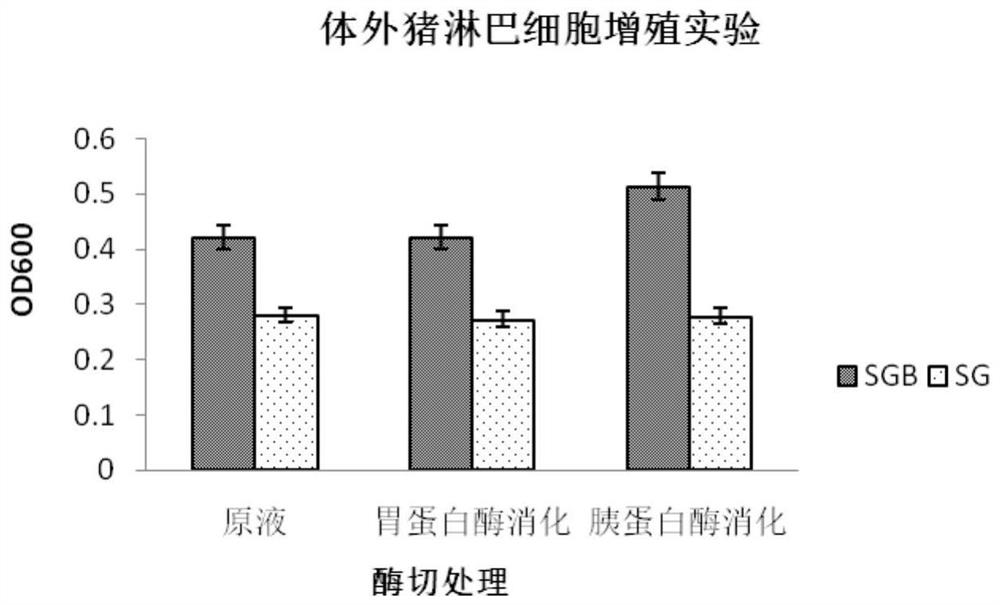
[0142] Embodiment 2, the influence of bovine antimicrobial peptide fusion protein (FBAP) on lymphocyte proliferation
[0143] 1. Preparation of recombinant bacteria SMDpG-B fermentation supernatant
[0144] (1) the recombinant bacterium SMDpG-B (hereinafter referred to as SGB) that embodiment 1 obtains is inoculated in 3mL substratum 1 (substratum 1 is the liquid medium that adds bleomycin (Zeocin) to obtain in YPD substratum, and The concentration of bleomycin was 100mg / mL), and the activated strain was cultivated overnight at 28°C and 200rpm.
[0145] (2) Take 300 μL of the bacterial solution obtained in step (1) and inoculate it into a 100 mL Erlenmeyer flask containing 30 mL of YPD medium, ferment at 28 °C and 200 rpm on a shaker for 48 hours (OD 600 for 25).
[0146] (3) Take 5 mL of the bacterial liquid obtained in step (2), centrifuge at 12 000×g for 2 min, and name the obtained supernatant as SGB fermentation supernatant.
[0147] 2. Protease treatment of the fermen...
Embodiment 3
[0164] Embodiment 3, the antibacterial activity detection of bovine antimicrobial peptide fusion protein (FBAP)
[0165] Determination of bovine antimicrobial peptide fusion protein FBAP on Escherichia coli standard bacteria (G - ) (hereinafter referred to as S-G - ), Escherichia coli resistant bacteria (G - ) (hereinafter referred to as R-G - ), Staphylococcus aureus standard bacteria (G + ) (hereinafter referred to as S-G + ), drug-resistant Staphylococcus aureus (G + ) (hereinafter referred to as R-G + ) antibacterial situation, the specific method is as follows:
[0166] First, the four bacterial strains were inoculated and activated and cultured in the exponential growth phase (OD 600 about 0.5), then diluted to OD with LB medium 600 About 0.005, the diluted bacterial solution was inoculated on a 96-well cell culture plate, 100 μL / well, one kind of bacteria per 96-well cell culture plate.
[0167] For each 96-well cell culture plate containing bacteria, it was pr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com