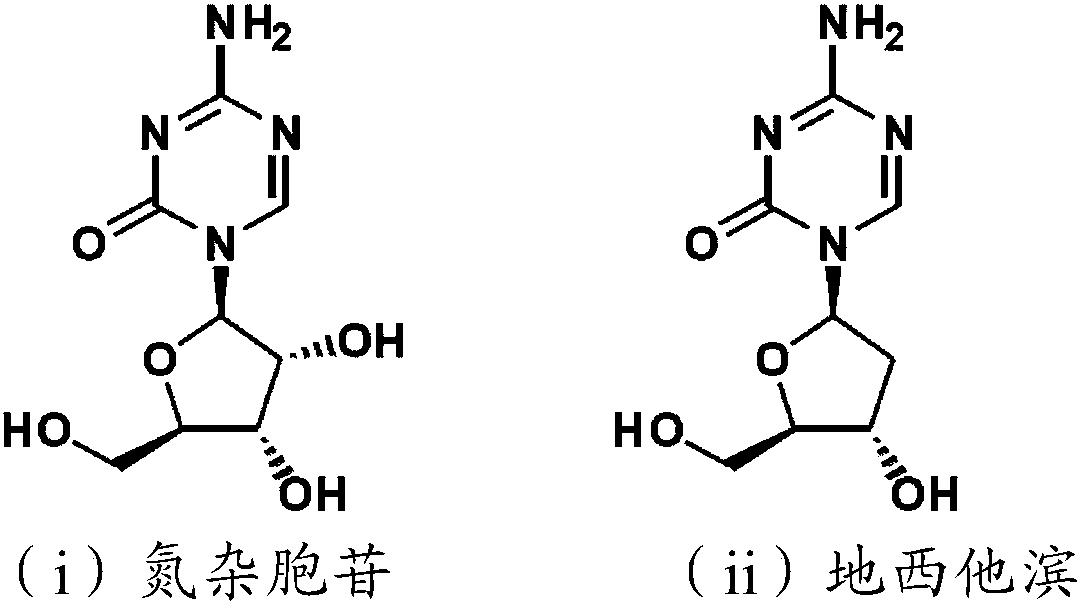
5'-position dibenzyl phosphoric acid ester of 5-azacytidine or 2'-deoxy body thereof
An azacytidine and benzyl technology, which is applied in the field of novel 5'-dibenzyl phosphate compounds, can solve the problems that the evaluation of the stability of the chemical structure cytidine deaminase and the biological activity has not been reported.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
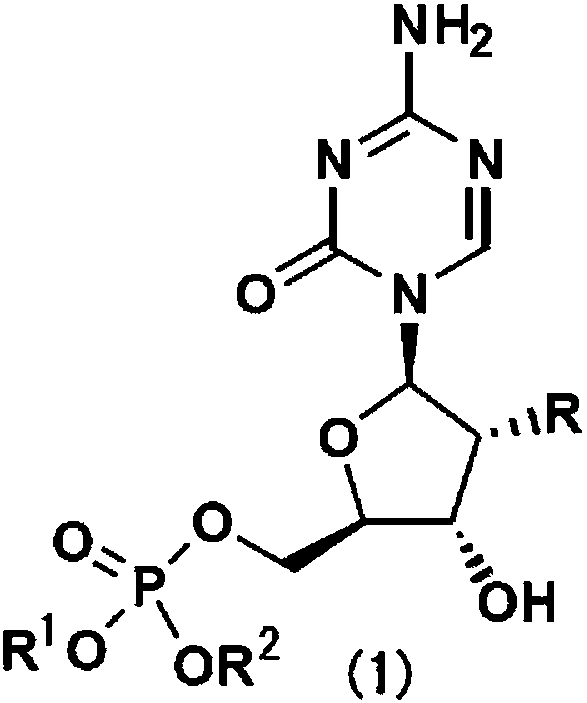
[0068] The preparation method of compound (1) of the present invention
[0069] For example, compound (1) of the present invention can be produced according to the following method or other similar methods.
[0070] Method A
[0071] Can according to routine method or other similar methods (refer to Bulletin of the Chemical Society, 1969,42 (12), 3505-8, Nucleic Acids Research, 1984, 12, 5025-36, Chemical & Pharmaceutical Bulletin, 1995, 43 (2), 210 -215, and WO-2011113173) to prepare compound (1) or a salt thereof. For example, commercially available 5-azacytidine or 2'-deoxy-5-azacytidine is activated by phosphorus oxychloride in a suitable solvent, and then reacted with benzyl alcohol which may have a substituent in the presence of a base . 5'-dibenzyl phosphate of 5-azacytidine as the target compound can be obtained (see chemical formula (1)).
[0072] Method B
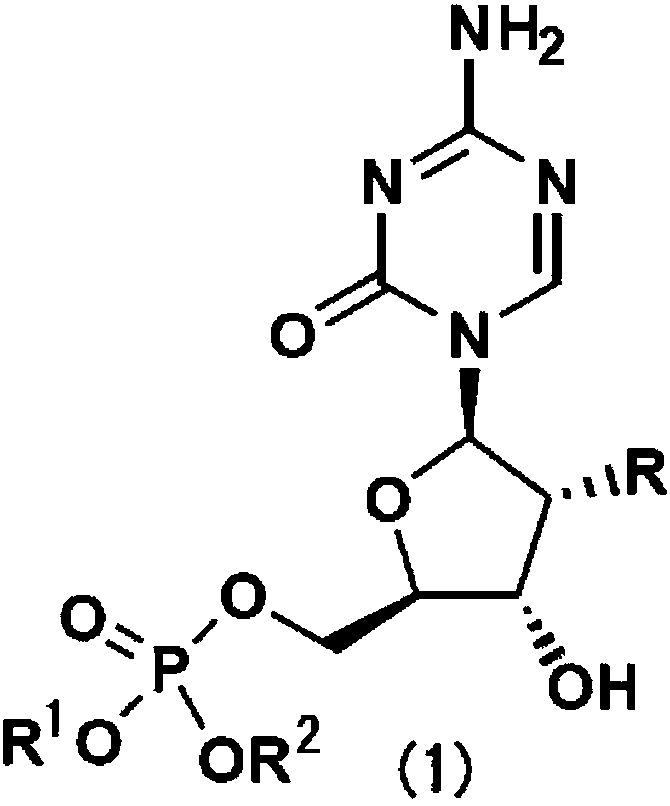
[0073] It can be prepared, for example, by reacting commercially available 5-azacytidine or 2'-deoxy...
Embodiment 1
[0101] Activation of 5-azacytidine with phosphorus oxychloride and subsequent condensation with benzyl alcohol
[0102] Suspend 122 mg of 5-azacytidine in about 1.0 mL of triethyl phosphate solution. When cooled to 0° C., 93 μL of phosphorus oxychloride (about 2.0 times that of 5-azacytidine as a starting material in terms of mol) was added, and stirred for about 1 hour. Then, about 0.5 mL (about 5 times in mol) of the corresponding benzyl alcohol and about 0.4 mL (about 9 times in mol) of pyridine were added to the solution, and stirred at 0° C. for another 1 hour. The reaction solution was poured into an ethyl acetate / water mixture, and neutralized with dilute sodium bicarbonate solution before extraction with ethyl acetate. The extract was washed with saturated brine, and dried over anhydrous magnesium sulfate. After removing insoluble matter by suction, the extract was concentrated to dryness under reduced pressure. The obtained oily residue was separated and purified w...
Embodiment 2
[0104] Condensation of 5-azacytidine with dibenzyl phosphorochloride derivatives
[0105] Suspend 122 mg of 5-azacytidine in 1.0 mL of anhydrous pyridine. When cooled to 0 °C, about 0.25 mL (about 1.2 times in mol) of the corresponding dibenzyl chlorophosphate derivative was added and stirred for about 1 hour. The reaction solution was poured into an ethyl acetate / water mixture, and neutralized with dilute sodium bicarbonate solution before extraction with ethyl acetate. The extract was washed with saturated brine, and dried over anhydrous magnesium sulfate. After removing insoluble matter by suction, the extract was concentrated to dryness under reduced pressure. The obtained oily residue was separated and purified with a silica gel column (Yamazen Smart Flash MS system) to obtain a 5'-dibenzyl phosphate derivative of 5-azacytidine as the target compound. This is referred to as Synthesis Method B hereinafter.
[0106] The 5'-dibenzyl (or diphenylethyl, diphenyl) phosphate...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com