Selective fluorination method for isatin hydrazones compounds
A compound, the technology of isatin hydrazone, which is applied in the field of synthesis of fluorine-containing drug intermediates, can solve the problems of poor substrate adaptability, harsh reaction conditions, and low yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
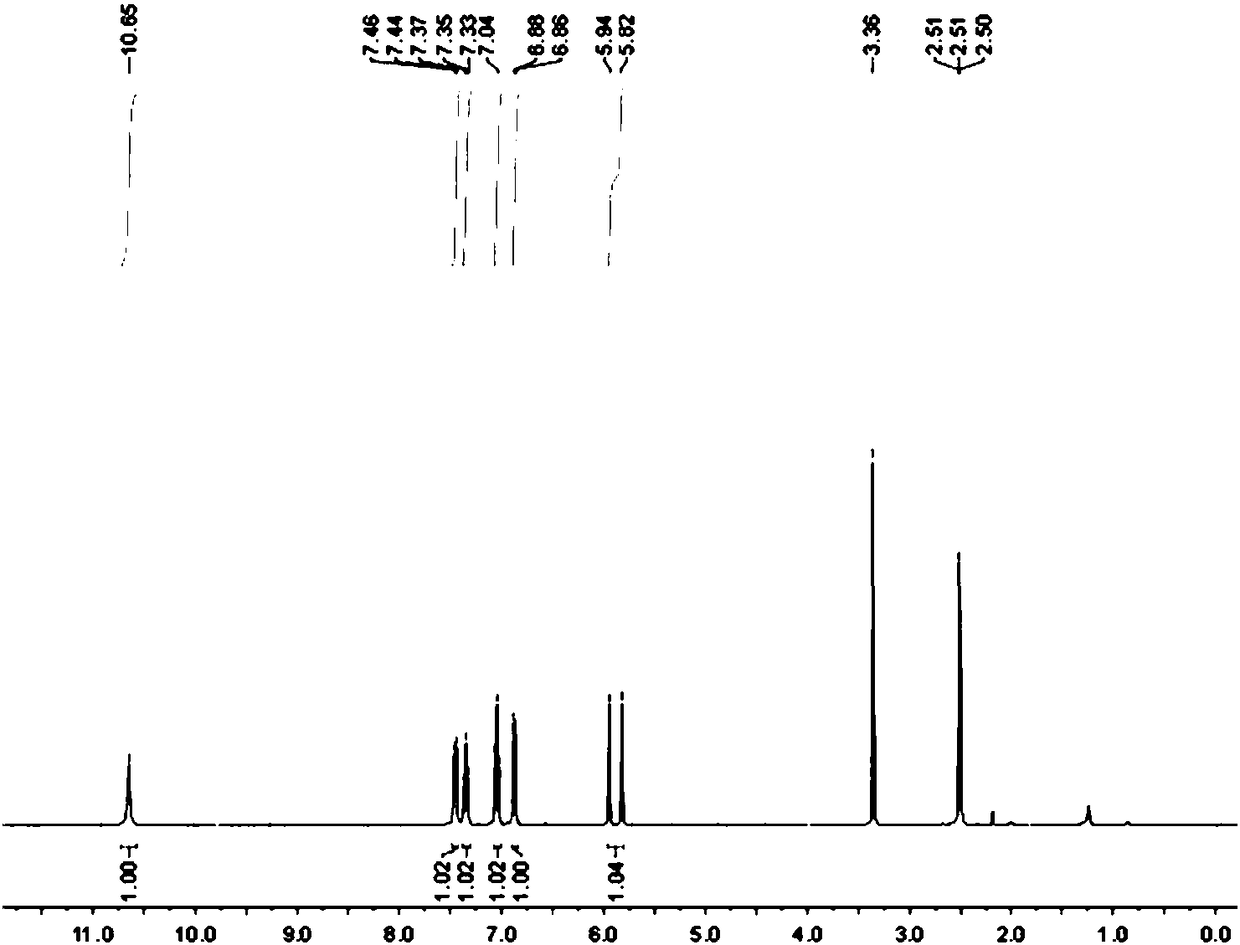
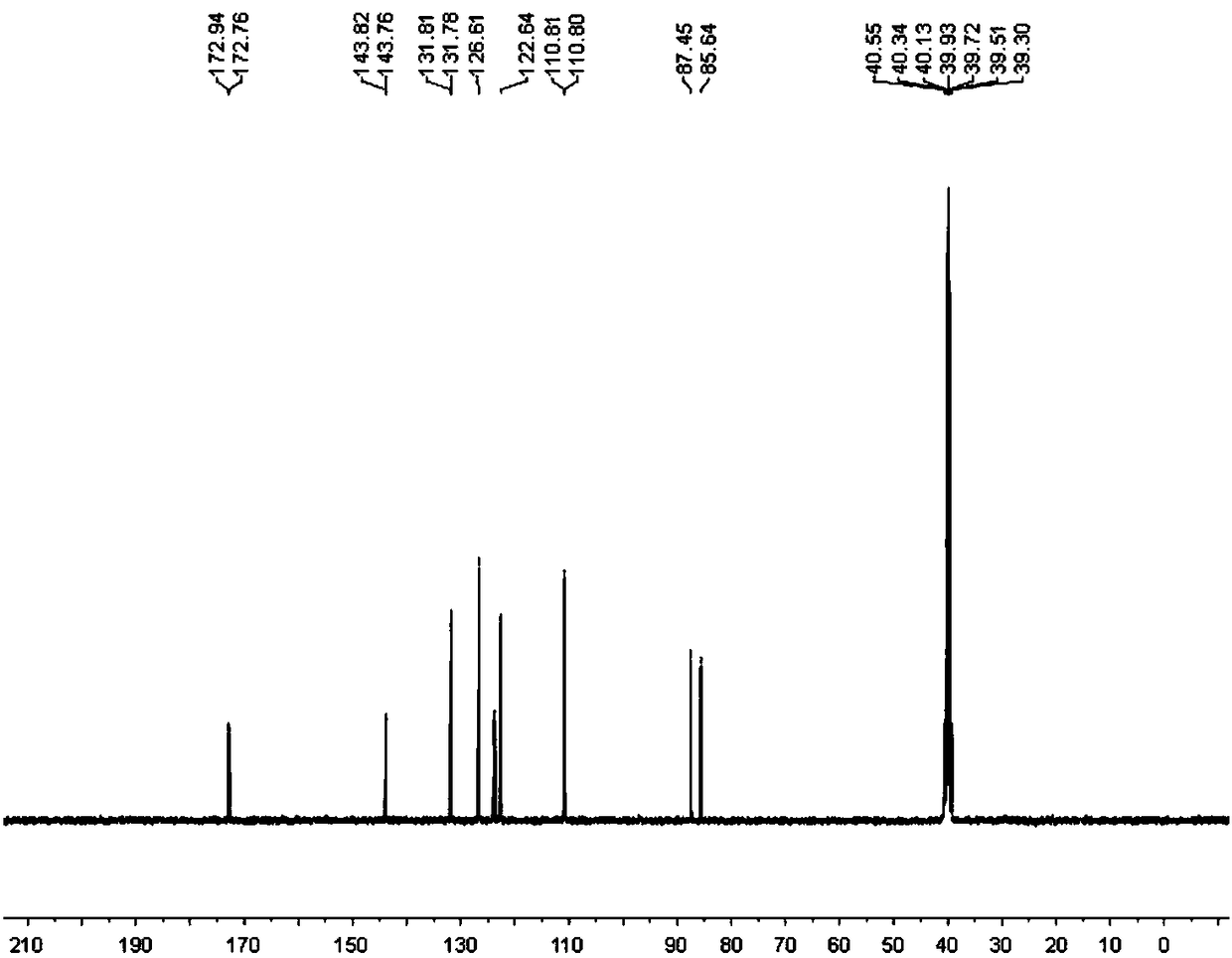
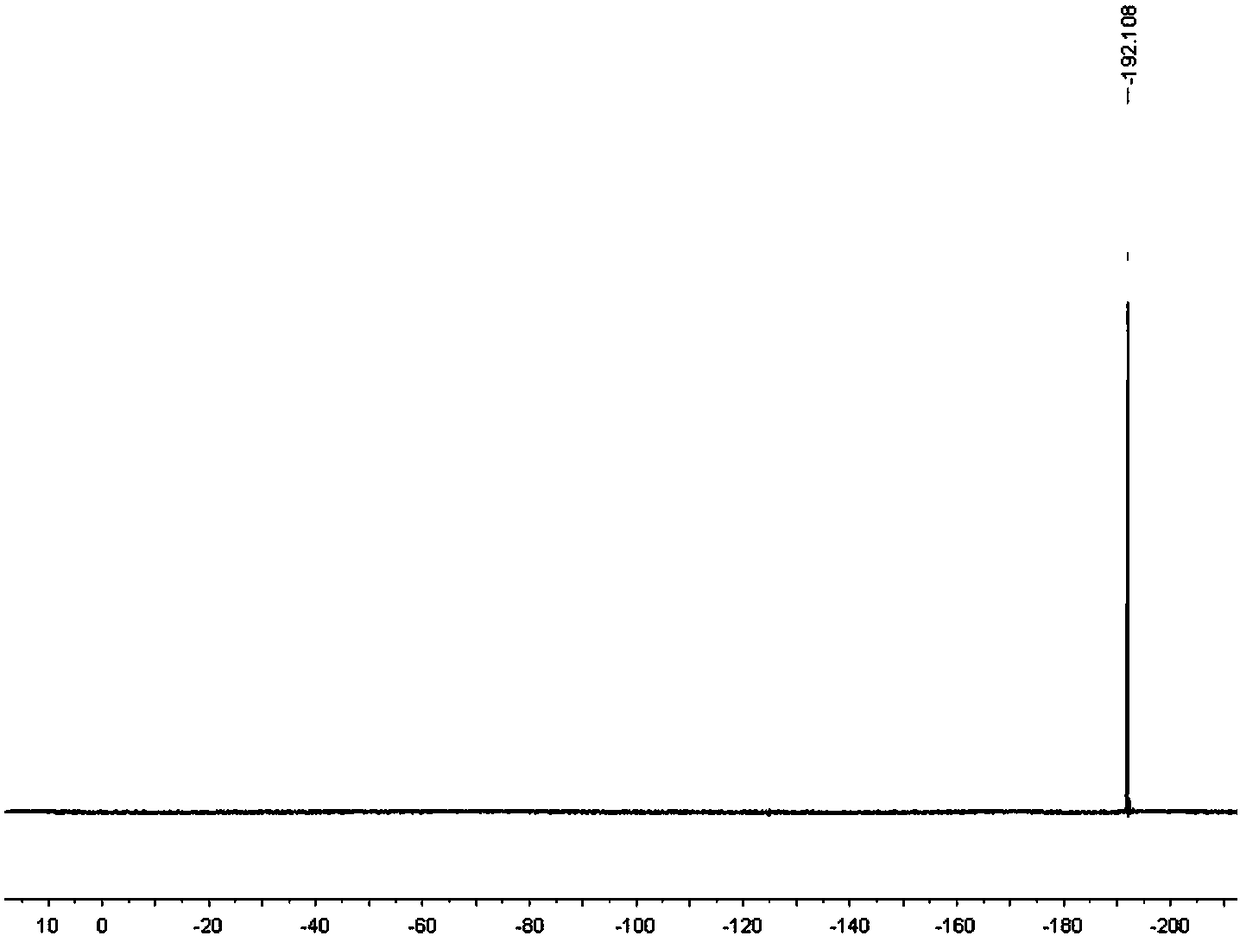
[0084] Synthesis, separation and purification of 3-fluoro-2-indolinone: add isatin hydrazone (0.5mmol, 1eq), Selectfluor (354.3mg, 1mmol, 2eq), acetic acid in a 100mL round bottom flask equipped with a magnetic stirrer Lithium (197.8mg, 3mmol, 6eq) was sealed with a rubber stopper; then ethylene dichloride (13mL) was added with a syringe as a solvent; the reaction bottle was placed in an oil bath at 70°C and heated and stirred for 18 hours. Layer chromatography (TLC) monitoring. After the reaction was completed, it was cooled to room temperature, and the reaction mixture was extracted twice with 40 mL of ethyl acetate, the organic phases were combined and washed with saturated brine, and then anhydrous sodium sulfate was added to dry the organic phase. After drying, the organic phase was spin-dried to dry the organic solvent under reduced pressure to obtain a crude product. The crude product was separated by column using 9:1 petroleum ether and ethyl acetate as eluent to obta...
Embodiment 2
[0089] Synthesis, separation and purification of 3-fluoro-1-isopropyl-2-indolone: 1-isopropyl-isatin hydrazone (0.5mmol, 1eq) was added to a 25mL round bottom flask equipped with a magnetic stirrer , Selectfluor (354.3mg, 1mmol, 2eq), lithium acetate (197.8mg, 2mmol, 6eq) and seal with a rubber stopper; then add dichloroethane (3mL) as a solvent with a syringe; the reaction bottle is placed in an oil bath at 80°C The reaction was heated and stirred in a pot for 16 hours, and the reaction progress was monitored by thin-layer chromatography (TLC). After the reaction was completed, it was cooled to room temperature, and the reaction mixture was extracted twice with 20 mL of ethyl acetate, the organic phases were combined and washed with saturated brine, and then anhydrous sodium sulfate was added to dry the organic phase. After drying, the organic phase was spin-dried to dry the organic solvent under reduced pressure to obtain a crude product. The crude product was separated b...
Embodiment 3
[0095] Synthesis, separation and purification of 3-fluoro-1-allyl-2-indolinone: 1-allyl-isatin hydrazone (0.5mmol, 1eq) was added to a 25mL round bottom flask equipped with a magnetic stirrer , Selectfluor (354.3mg, 1mmol, 2eq), lithium acetate (197.8mg, 2mmol, 6eq) and seal with a rubber stopper; then add dichloroethane (3mL) as a solvent with a syringe; the reaction bottle is placed in an oil bath at 80°C The reaction was heated and stirred in a pot for 16 hours, and the reaction progress was monitored by thin-layer chromatography (TLC). After the reaction was completed, it was cooled to room temperature, and the reaction mixture was extracted twice with 20 mL of ethyl acetate, the organic phases were combined and washed with saturated brine, and then anhydrous sodium sulfate was added to dry the organic phase. After drying, the organic phase was spin-dried to dry the organic solvent under reduced pressure to obtain a crude product. The crude product was separated by column...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com